Scientific papers
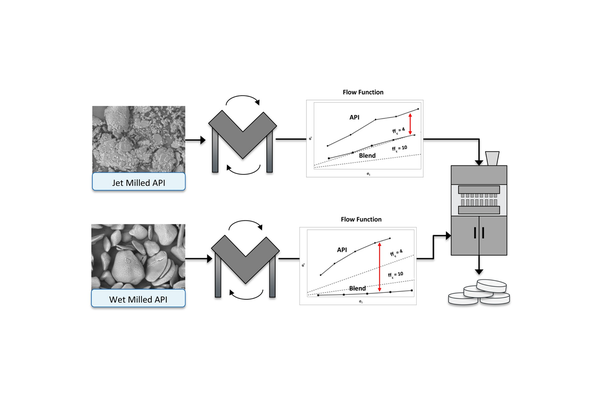
Optimizing the powder flow and compaction properties is crucial to ensure a reliable tablet manufacturing process. The significance of the flow and compaction properties of the active pharmaceutical ingredient (API) becomes more pronounced in higher drug load formulations and during the scaling up of manufacturing processes. This study illustrates that the flow properties of a powder blend can be enhanced through API particle engineering without significantly affecting blend tabletability at elevated drug loadings. When examining a jet-milled API (D50 = 24 μm) and a particle-engineered wet-milled API (D50 = 70 μm and 90 μm), it was observed that the flow functions of all API lots were similarly poor, despite the substantial difference in average particle size (ffc < 4). This discovery challenges the conventional belief that powder flow properties are directly linked to particle size distribution. However, upon the addition of excipients, clear trends in flow functions based on API particle size emerged. Wet-milled API blends exhibited significantly improved flow functions (ffc > 10) compared to the jet-milled API blends. Investigation into the compaction properties of both wet and jet-milled powder blends also revealed that both materials produced robust tablets at the utilized drug loadings. Demonstrating this unusual observation, where similarly poor-flowing APIs can exhibit a marked difference upon blending, holds significant importance in pharmaceutical development. This is particularly relevant in early-phase development during API selection and proves advantageous, especially when employing material-sparing techniques.
Comments
No comments posted yet.
Add a comment