Scientific papers
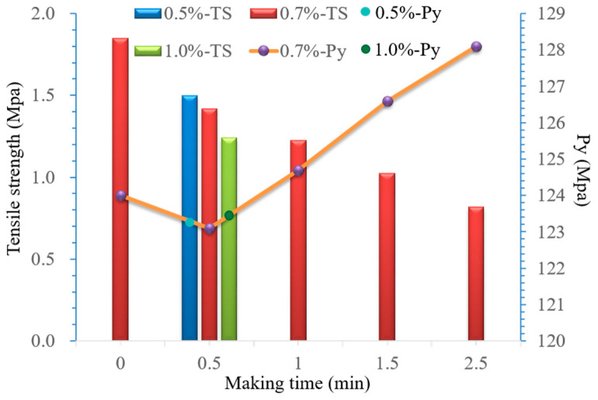
Hydrochlorothiazide (HCTZ)/losartan potassium (LOS-K) served as a model drug for the preparation of compound tablets. The investigation focused on the compression and mechanical properties of mixed powders to identify formulation and preparation factors. A D-optimal mixture experimental design was then employed to optimize the final parameters. The type and quantity of lactose monohydrate (SuperTab®14SD, 19.53-26.91%), microcrystalline cellulose (MCC PH102, 32.86-43.31%), pre-gelatinized starch (Starch-1500, 10.96-15.91%), and magnesium stearate (0.7%) were determined based on compressive work, stress relaxation curves, and Py value.
The compression mechanism of the mixed powder was further explored using the Kawakita equation, Shapiro equation, and Heckel analysis, categorizing the mixed powder as a Class-II powder. Recommended compaction pressure (150-300 MPa) and tableting speed (1200-2400 Tab/h) were identified. Utilizing a D-optimal mixture experimental design, the optimal formulation (No 1, comprising 26.027% lactose monohydrate, 32.811% MCC PH102, and 15.462% pregelatinized starch) was selected based on the drug dissolution rate, using Hyzaar® tablets as a control.
Upon oral administration in beagle dogs, there were no significant differences in bioavailability between the No. 1 tablet and the Hyzaar® tablet in terms of HCTZ, losartan carboxylic acid (E-3174), and LOS-K (F < F0.05). This approach, which considers a combination of compression and mechanical properties of mixed powder along with tablet quality, proves to be a viable method for direct powder compression.
Comments
No comments posted yet.
Add a comment