Scientific papers
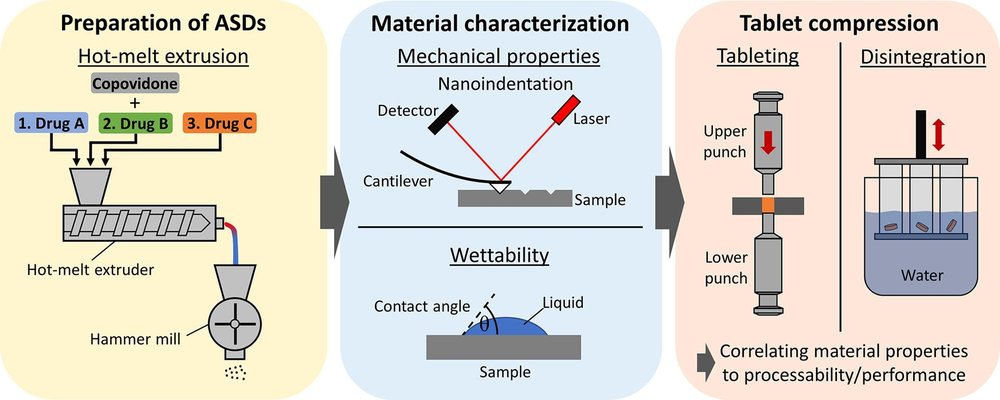
Despite the widespread use of amorphous solid dispersions (ASD) for poorly water-soluble drugs in the market, the formulation development of ASDs remains challenging. In the process of developing novel drugs, the conventional approach involves selecting the preparation technique and polymer matrix for a specific drug through screening tools. However, when investigating general trends related to material properties, this approach proves less beneficial, despite its frequent use in literature. The polymer is typically the main component of ASDs, predominantly determining the material properties of the system. To examine the impact of different drugs and their drug loads on mechanical properties and wettability, three poorly soluble model drugs with drug loads ranging from 10% to 40% were incorporated into copovidone through hot-melt extrusion.
The resulting extrudates underwent characterization for mechanical properties using diametral compression tests and nanoindentation, and the results were compared to performance during tablet compression. The inclusion of all tested drugs led to a consistent increase in the brittleness of the ASDs, while the Young’s modulus and hardness exhibited varying changes depending on the incorporated drug. These observations aligned well with the tablet compression performance, suggesting that brittleness appeared to be the predominant factor influencing the compression behavior of copovidone-based ASDs. Additionally, the degree of water absorption and wettability was assessed through dynamic vapor sorption experiments and contact angle measurements. The incorporated drugs influenced the contact angle to different extents, and a strong correlation between the contact angle and disintegration time was observed. These findings underscore the significance of thorough characterization of ASDs, aiding in predicting their performance during tablet compression and facilitating the optimal selection of excipients.
Comments
No comments posted yet.
Add a comment