Scientific papers
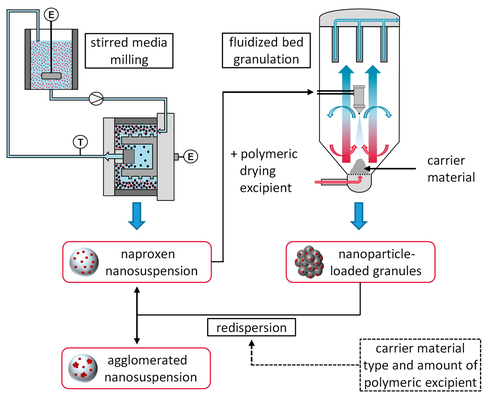
Reducing the particle size of active pharmaceutical ingredients is an efficient strategy for addressing challenges related to poor aqueous solubility. To enhance stability and patient convenience, nanosuspensions are often converted into solid dosage forms. This study explored the impact of various formulation parameters (carrier material type, type and quantity of additional polymeric drying excipient in the nanosuspension) on the redispersibility of naproxen nanoparticle-loaded granules produced in a fluidized bed process. The dissolution rate of the carrier material (sucrose, mannitol, or lactose) was identified as a crucial material property, where higher dissolution rates (sucrose > mannitol > lactose) led to better redispersibility. Moreover, increasing amounts of polymeric drying excipient in the nanosuspension were associated with improved redispersibility. The correlation between redispersibility and the degree of nanoparticle embedding on the granule surface was observed, likely resulting from partial dissolution and subsequent resolidification of the carrier surface, dependent on the carrier material's dissolution rate, or resolidification of the dissolved polymeric drying excipient during drying. As this correlation held for all investigated formulation parameters, it is suggested that the redispersibility of nanoparticles is influenced by their arrangement in the dried state.
Comments
No comments posted yet.
Add a comment