Scientific papers
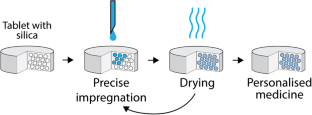
The production of personalized medicines, where specific combinations of active pharmaceutical ingredients (APIs) and their doses within a tablet are adjusted to individual patient needs, necessitates novel manufacturing approaches compared to conventional practices. Particularly in low-dose formulations, achieving the required precision of API content may be challenging using traditional unit operations like solid powder blending. This study aims to explore an alternative approach based on the concept of pre-formulated placebo tablets containing mesoporous silica particles capable of absorbing APIs in solution form, allowing for precise dosing even at extremely low quantities. The accuracy of the liquid dosing system was validated, demonstrating satisfactory mechanical properties of the tablets after multiple impregnation-drying cycles and the ability to meet pharmacopoeia specifications on content uniformity. Using model APIs, the spatial distribution of the API within the tablet post-impregnation was investigated, revealing a dependence on the number and order of impregnation-drying cycles. It was observed that loading an API to the tablet in a single step yielded a different dissolution profile compared to the same quantity dosed in multiple smaller steps. Overall, the approach of loading multiple APIs to a pre-formulated tablet at defined quantities using drop-on-demand liquid dosing was deemed feasible from the perspective of dose uniformity. Future research should delve into potential API interactions and the storage stability of tablets manufactured using this method.
Comments
No comments posted yet.
Add a comment