Scientific papers
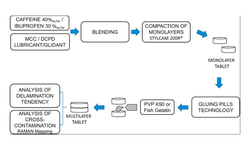
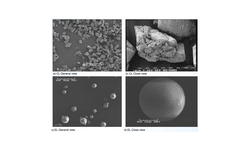
A fixed-dose combination tablet formulation of drugs Y and Z was developed using a bilayer tablet approach. Layer I, containing 250 mg of Drug Y, was produced through dry granulation, while Layer II A or II B, containing 1280 mg of Drug Z, employed a moisture-activated dry granulation (MADG) process. Both Layer II A and Layer II B included 3% w/w colloidal silicon dioxide (Aeroperl® 300 with a surface area of 300 m2/g, and Aerosil® 200 with a surface area of 200 m2/g, respectively) for moisture scavenging, along with other common excipients. Both grades of silicon dioxide were amorphous.
Upon exposure to an open relative humidity of 40°C/75% for 72 hours, the bilayer tablet with Layers I/Layer II A (containing Aeroperl® 300) exhibited clear layer separation, whereas the tablet with Layers I/Layer II B (containing Aerosil® 200) did not. Under similar conditions, the projected change in moisture content for Layer I, Layer II A, and Layer II B could be 63% w/w, 107% w/w, and 109% w/w, respectively. The difference in moisture adsorption between Layers I/Layer II A (Aeroperl® 300) and Layers I/Layer II B (Aerosil® 200) was similar.
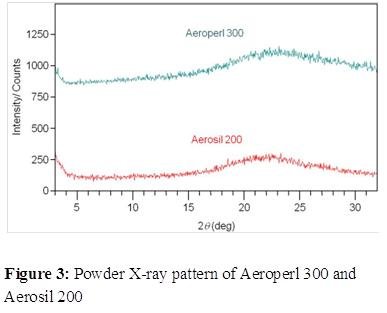
Comparing the moisture adsorption-desorption isotherms for Aeroperl® 300 and Aerosil® 200 indicated that Aeroperl® 300 can adsorb larger amounts of moisture at any humidity level due to its greater surface area but does not retain moisture as humidity decreases. In contrast, Aerosil 200 adsorbs relatively smaller amounts of moisture but retains it due to its larger pore sizes. It is hypothesized that the moisture not retained by Aeroperl® 300 could interact with other Layer I excipients, such as microcrystalline cellulose and crospovidone, generating significant shear stress at the layer interface and triggering delamination.
Comments
No comments posted yet.
Add a comment