Scientific papers
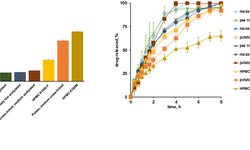
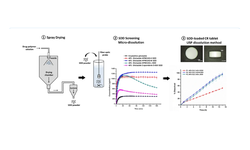
The controlled release of active ingredients like chlorzoxazone from matrix tablets, utilizing Kollidon®SR and chitosan, relies on both the drug's solubility in the dissolution medium and the composition of the matrix. This study aims to develop new oral matrix tablet formulations, utilizing Kollidon®SR and chitosan, to enhance the oral bioavailability of chlorzoxazone, a class II non-steroidal anti-inflammatory drug according to the Biopharmaceutical Classification System. Nine variations of chlorzoxazone matrix tablets were produced through direct compression, adjusting the ratio of components as follows: 1:1, 1:2, and 1:3 chlorzoxazone/excipients, 20–40 w/w % Kollidon®SR, 3–7 w/w % chitosan, while keeping auxiliary substances (Aerosil® 1 w/w %, magnesium stearate 0.5 w/w %, and Avicel® up to 100 w/w %) constant. The tablets underwent pharmacotechnical characterization, including analysis of flowability and compressibility properties (flow time, friction coefficient, angle of repose, Hausner ratio, and Carr index), and pharmacochecmical characteristics (mass and dose uniformity, thickness, diameter, mechanical strength, friability, softening degree, and in vitro release profiles). Based on the results, three formulations (F1b, F2b, and F3b) with 30 w/w % Kollidon®SR and 5 w/w % chitosan were selected for further investigation. These formulations underwent detailed analysis using Fourier-transform infrared spectrometry, X-ray diffraction, thermogravimetry, and differential scanning calorimetry. Comparative studies on the release kinetics of active ingredients were conducted through in vitro release testing, with results analyzed using four mathematical models for modified-release oral formulations. The kinetic study revealed a two-step release mechanism occurring within 0–2 hours and 2–36 hours. The release profile of chlorzoxazone was assessed using f1 (similarity factor) and f2 (difference factor). Findings indicated that both Kollidon®SR and chitosan are effective matrix-forming agents in combination with chlorzoxazone. The three selected formulations exhibited optimal pharmacotechnical properties and in vitro kinetic behavior, suggesting their potential for use in oral pharmaceutical products for the controlled delivery of chlorzoxazone. Dissolution tests demonstrated faster drug release for the F2b sample.
Comments
No comments posted yet.
Add a comment