Scientific papers
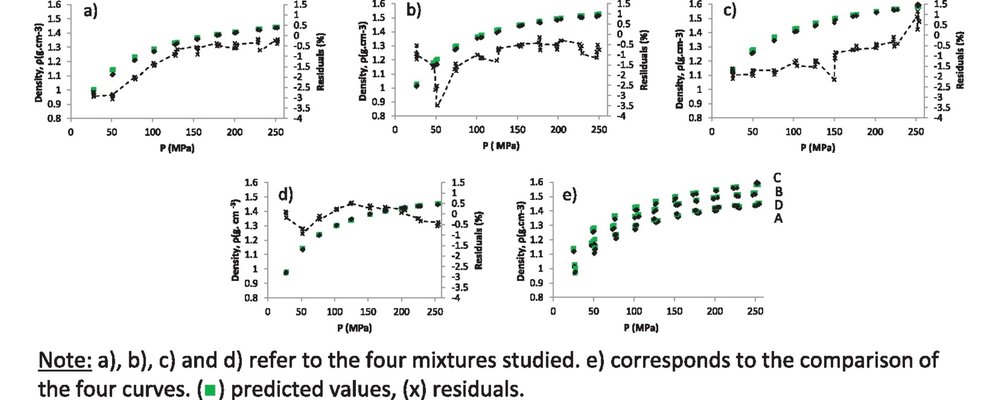
The development of predictive models for the pharmaceutical compaction process holds significant importance, not only in the formulation stage but also within the framework of Quality by Design (QbD) development. This paper focuses on predicting compressibility, specifically forecasting the changes in density and porosity of the compact in response to compaction pressure, both "in-die" (during compaction) and "out-of-die" (post ejection of the compact). To achieve this, a rotative press simulator was employed to study four different mixtures consisting of five distinct pharmaceutical products. The selection of excipients and formulations aimed to closely resemble real industrial formulations.
Utilizing volume as an additive property and a reformulated version of the Kawakita equation based on density, accurate predictions of compact density, both "in-die" and "out-of-die," were achieved (residuals <3.5%). For the pressure levels typical in the pharmaceutical industry, the absolute error in predicting porosity was generally below 2%. This study underscores the potential suitability of this approach for predicting the compressibility of authentic pharmaceutical formulations in an industrial context.
Comments
No comments posted yet.
Add a comment