Scientific papers
This study aims to explore the challenges associated with incorporating Vitamin E TPGS (d-α tocopheryl polyethylene glycol 1000 succinate) into solid pharmaceutical dosage forms. Employing a wet granulation process involving high-shear and fluid bed techniques, Vitamin E TPGS was introduced as part of the binder solution during granulation. The findings indicate that Vitamin E TPGS can be successfully integrated into a prototype formulation at a concentration of 10% w/w without encountering significant processing challenges. However, the resulting granulations exhibited successful compression only at low tablet press speeds (dwell time ~100 ms). Under conditions reflecting commercial tablet manufacturing (dwell times <20 ms), a notable reduction in compactability was observed, accompanied by various tablet defects.
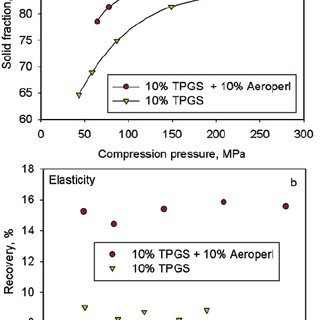
To address these compaction issues, intragranular incorporation of Aeroperl® 300, a granulated form of colloidal silicon dioxide, proved effective. The formulation containing Aeroperl® 300 demonstrated a considerable decrease in granule particle size, an increase in granule porosity, and enhanced compactability compared to the formulation without Aeroperl® 300.
Comments
No comments posted yet.
Add a comment