Scientific papers
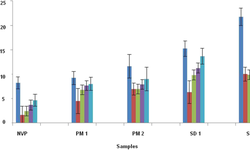
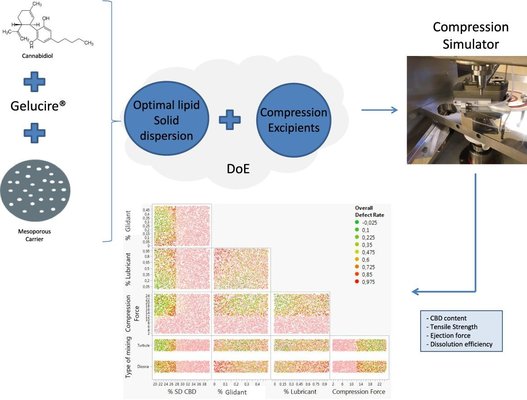
This study pursued dual objectives. Firstly, it sought to identify the most effective lipid solid dispersion for cannabidiol from a range of lipid excipients (Gelucire® 50/13, 48/16, 44/14, and Labrasol®) and inorganic carriers (colloidal silica, Syloid® XDP, and Neusilin® US2) through a screening plan. The primary aim was to improve the aqueous solubility of cannabidiol, resulting in a free-flowing powder with sufficient drug content. This involved combining cannabidiol (20%) with Gelucire® 50/13 (40%; Gattefossé, France), encapsulated within the mesopores of mesoporous silica Syloid® XDP (40%; Grace, Germany).
Secondly, the study explored the tableting properties of the chosen dispersion using a Design of Experiments (DoE) approach. Tablets were produced with additional excipients, and a compression simulator (Styl’One® Evo, Medelpharm, France) was utilized. The DoE considered variables such as the percentage of lipid solid dispersion, glidant, lubricant, and various compression forces. Key parameters, including dissolution efficiency, drug content, tensile strength, and ejection force, were scrutinized.
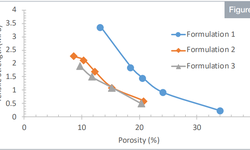
The DoE outcomes underscored the significance of both the percentage of dispersion and compression forces as influential variables. Notably, the study brought attention to a phenomenon where lipid materials exited the mesopores of silica during the compression process, resulting in reduced tensile strength. While demonstrating the feasibility of manufacturing tablets with lipid materials, the study identified specific limitations. In particular, the dispersion percentage should not exceed 27%, and compression forces of up to 13 kN are necessary to produce lipid tablets with optimal properties.
Comments
No comments posted yet.
Add a comment