Scientific papers
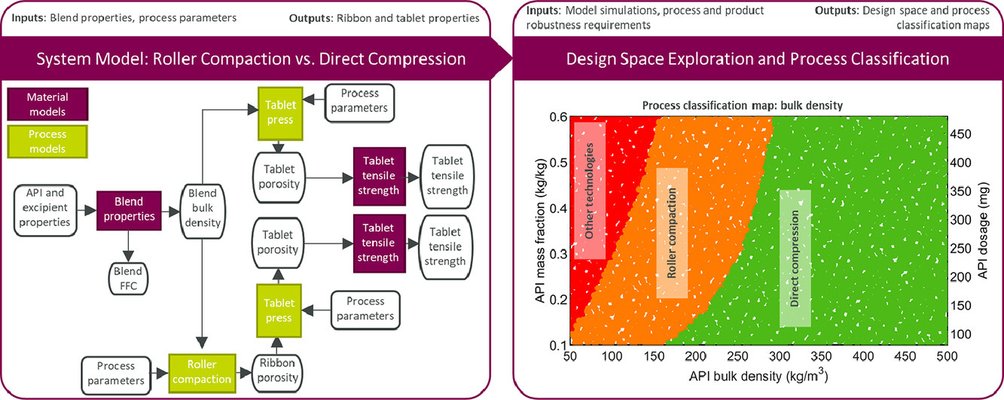
Utilizing mechanistic process modeling offers an opportunity to alleviate experimental demands, allowing for the establishment of relationships between process parameters and product attributes through in-silico experiments. A system model for a pharmaceutical tablet manufacturing process, comparing dry granulation with direct compression, has been developed to address crucial material and process design inquiries. This system model establishes links between API physical properties, formulation, and process parameters, enabling the delineation of a robust operating space.
To exemplify the model's application, various drug product formulation design questions were explored:
• Which processing route is the most robust based on API material properties and dosage requirements?
• How does drug loading and tablet size affect the manufacturing process's robustness?
• What process settings are necessary for a robust manufacturing route considering API material properties and drug loading requirements?
A computational framework was created using the system models to generate process classification and design space maps, facilitating robust decision-making in pharmaceutical formulation and process design. Process classification maps were generated to assess the feasibility of roller compaction and direct compression for different material properties and formulations. Constraints on critical quality attributes were defined using the Manufacturing Classification System. The design space maps showcased here illustrate how system models support formulation and process design by showcasing variations in the process operating space with changes in API mass fraction.
The system model for process design and selection demonstrates how understanding API physical properties allows for modeling the impact of formulation and process design. Moreover, these models prove valuable in discussions with colleagues developing the API to establish the necessary requirements for API physical properties, ensuring successful and robust formulation and process designs.
Comments
No comments posted yet.
Add a comment