Scientific papers
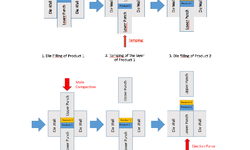
Tablet disintegration is a crucial prerequisite for drug dissolution and absorption. While the disintegration test conventionally takes place at 37 °C, intragastric temperature variations can occur due to meals or fever. This study delves into the impact of temperature and compaction pressure on tablet disintegratability, aiming to enhance our understanding of superdisintegrant sensitivity and functionality. Tablets containing either sodium starch glycolate or crospovidone as the disintegrant were crafted under varied compaction pressures and underwent disintegration testing at different medium temperatures. To establish instant temperature equilibrium, tablet preheating was employed between the tablet and the disintegration medium. Faster liquid penetration and disintegration occurred with higher medium temperatures or decreased compaction pressure. Disintegrants with swelling or strain recovery exhibited comparable sensitivity to medium temperature variations. Although tablet preheating led to slower disintegration, this effect was reversible upon cooling, suggesting it was unlikely due to changes in the disintegrant's physical state. The temperature difference between the tablet and the disintegration medium appeared to influence fluid flow rates into tablets, impacting disintegration. A comprehension of disintegrant temperature sensitivity can help prevent undesirable fluctuations in disintegration caused by temperature changes. Moreover, this temperature difference effect could potentially be leveraged to enhance disintegrant performance.
Comments
No comments posted yet.
Add a comment