Scientific papers
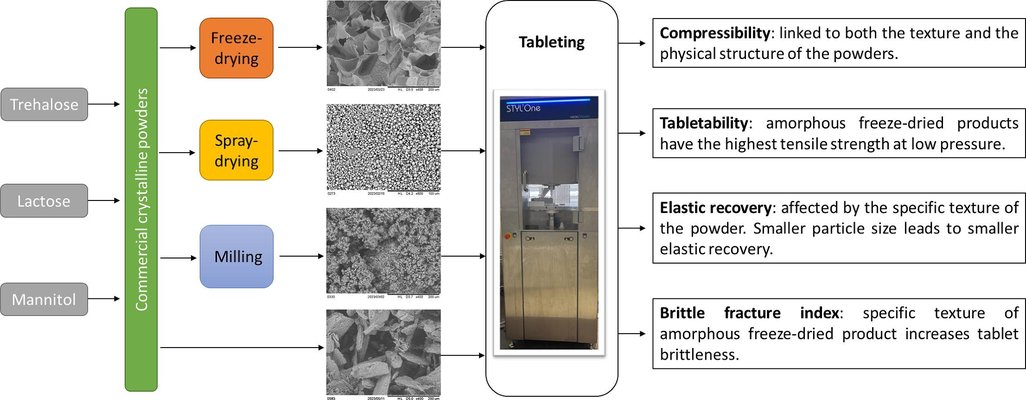
Liquid biopharmaceuticals often face stability challenges during storage, making their preservation a priority. Among the various drying techniques employed in the pharmaceutical industry to enhance stability, lyophilization stands out as the most prevalent. Additionally, for biopharmaceuticals intended for oral administration, tablets represent the primary solid dosage form. Hence, the tableting characteristics of freeze-dried products, serving as cryo and lyoprotectants, emerge as crucial for advancing pharmaceutical developments and applications. This study delves into the properties influencing the distinct compaction behavior of freeze-dried excipients. Specifically, we scrutinized the tableting properties of freeze-dried trehalose, lactose, and mannitol, contrasting them with other forms of these excipients (such as spray-dried, commercial crystalline, and commercial crystalline milled powders). The findings revealed unique compressibility, tabletability, and brittleness traits in the amorphous powders resulting from freeze-drying. Furthermore, the comparison with alternative powders underscored that this distinct tableting behavior is attributed to both the specific texture and the physical state (amorphization) of freeze-dried powders.
Comments
No comments posted yet.
Add a comment














