Scientific papers
"Excipients are widely present in pharmaceutical products, often playing a crucial role in maintaining product quality. In the case of products containing drugs sensitive to moisture, the presence of moisture can harm product stability during storage. Therefore, utilizing excipients that interact with moisture in the product can potentially alleviate stability issues.
This study focused on enhancing the interactive behavior of starch with moisture by coprocessing maize starch with sodium chloride (NaCl) or magnesium nitrate hexahydrate [Mg(NO3)2·6H2O] at varying concentrations (5 and 10%, w/w). The impact of these formulations on drug stability was evaluated by assessing the degradation of acetylsalicylic acid, serving as the model drug.
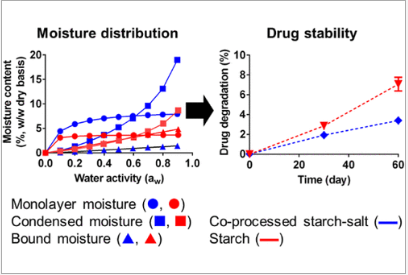
The findings revealed that coprocessing starch with either NaCl or Mg(NO3)2·6H2O influenced the number of water molecule binding sites on the starch and the distribution of sorbed moisture. Consequently, coprocessed excipients led to reduced drug degradation and minimized changes in tablet tensile strength during post-compaction storage. However, tablet formulations containing physical mixtures of starch and salts did not yield promising results.
This study underscores the advantageous simultaneous use of common excipients through coprocessing to synergistically counteract the adverse effects of moisture and enhance product stability when formulating moisture-sensitive drugs. Furthermore, these insights contribute to a better understanding of moisture–excipient interactions, aiding in the strategic selection of excipients for formulations involving moisture-sensitive drugs."
Comments
No comments posted yet.
Add a comment