Combining patient centricity and commercial viability in pediatric product development
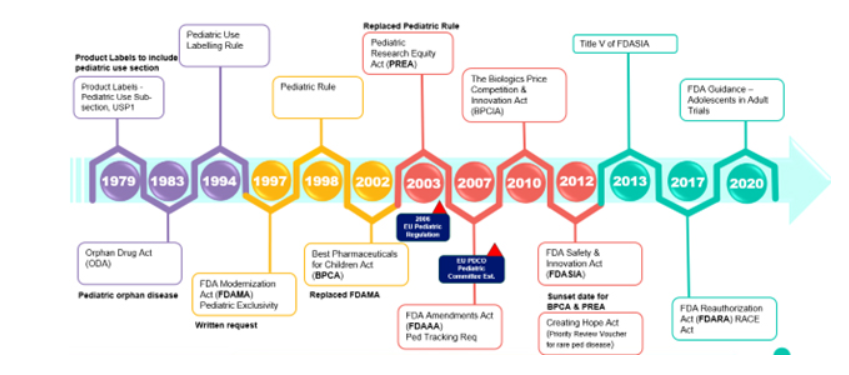
In pediatric drug formulation, achieving patient adherence and acceptance is crucial due to children’s rapid development and unique pharmacokinetic profiles. Pediatric formulations face specific challenges, including complex regulations, formulation demands, and small market size. As outlined by Srinivasan Shanmugam, Ph.D., of Adare Pharma Solutions, evolving regulations like the Pediatric Rule, BPCA, and PREA in the U.S. and the EU Pediatric Regulation mandate age-appropriate formulations, emphasizing safety and efficacy.
Central to pediatric product success is patient-centric design that considers taste, swallowability, and dosage flexibility to enhance therapeutic outcomes. Adare’s expertise in taste-masking and multiparticulate technologies, such as Microcaps® and Minitabs™, addresses these needs with innovative formats like orally disintegrating tablets and sprinkle beads, making medications easier to administer and more acceptable to young patients.
Shanmugam highlights how Adare’s solutions, including enteric-coated, taste-masked mini-tablets, meet complex pediatric dosing needs for conditions like cystic fibrosis. By combining regulatory compliance and patient-centricity, Adare offers tailored, marketable solutions that improve treatment outcomes for children worldwide.
Comments
No comments posted yet.
















