Scientific papers
In this study, the application of multiparticulate drug delivery systems (MDDS) was explored for tablets designed to amorphize supersaturated granular amorphous solid dispersions (ASDs) in situ—specifically, achieving amorphization through microwave irradiation within the final dosage form. The MDDS concept was proposed to ensure the geometric and structural stability of the dosage form and enhance in vitro disintegration and dissolution characteristics.
Granules were prepared in two sizes (small and large), containing the crystalline drug celecoxib (CCX) and polyvinylpyrrolidone/vinyl acetate copolymer (PVP/VA) with a 50% w/w drug load, along with sodium dihydrogen phosphate monohydrate as the microwave-absorbing excipient. These granules were then incorporated into an extra-granular tablet phase, composed of either microcrystalline cellulose (MCC) or mannitol (MAN) as the filler, along with the disintegrant crospovidone and the lubricant magnesium stearate.
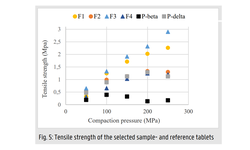
Tensile strength and disintegration time were investigated before and after 10 minutes of microwave irradiation (800 and 1000 W), and the resulting ASDs were characterized using X-ray powder diffraction and modulated differential scanning calorimetry. The internal structure was elucidated through X-ray micro-Computed Tomography (XµCT), and the dissolution performance of selected tablets was evaluated.
While the MDDS tablets exhibited no geometric changes after microwave irradiation, there was an increase in tensile strength and disintegration time. Complete amorphization of CCX was achieved only in the MCC-based tablets at a power input of 1000 W, whereas MAN-based tablets displayed partial amorphization regardless of power input. Complete amorphization was linked to the fusion of individual ASD granules within the tablets, impacting subsequent disintegration and dissolution performance. For these tablets, supersaturation was observed only after 60 minutes.
Conversely, partially amorphized MDDS tablets showed complete disintegration during dissolution experiments, leading to a rapid onset of supersaturation within 5 minutes and an approximately 3.5-fold degree of supersaturation within the experimental timeframe (3 hours). Overall, the MDDS concept demonstrated potential as a feasible dosage form for in situ amorphization, but further improvements are needed to achieve a fully amorphous and rapidly disintegrating system.
Comments
No comments posted yet.
Add a comment