Effects of omitting titanium dioxide from the film coating of a pharmaceutical tablet
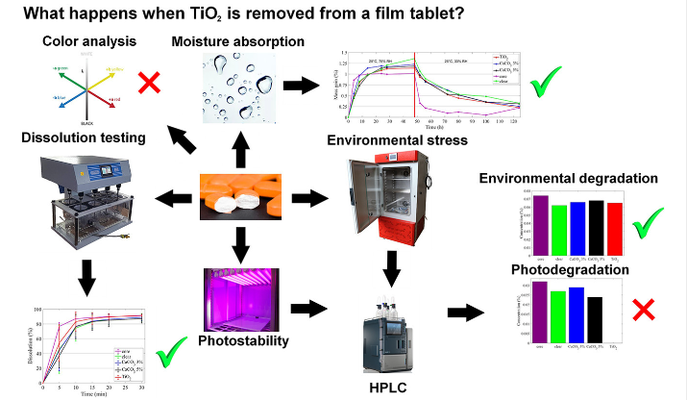
This case study investigates the impact of removing titanium dioxide (TiO2) from the film coating of pharmaceutical tablets, in light of impending EU regulations banning TiO2 in pharmaceuticals. The study evaluates the performance of alternative coatings using calcium carbonate (CaCO3) and transparent coatings. Key findings include:
*Moisture Absorption and Dissolution: No significant differences were found between the TiO2, CaCO3, and transparent coatings in terms of moisture absorption and dissolution rates.
*Photostability and Environmental Stability: For a highly photosensitive drug, TiO2 was crucial for protecting against light-induced decomposition. The absence of TiO2 led to increased degradation under light exposure, though there was no additional benefit from TiO2 under humidity and thermal stress.
*Tablet Composition and Testing: Tablets contained microcrystalline cellulose, lactose monohydrate, crospovidone, magnesium hydroxide, and magnesium stearate. The study tested coatings with different weight gains of TiO2, CaCO3, and a clear coating, revealing that thicker CaCO3 coatings did not compensate for the lack of TiO2's light protection.
*Coating Process: Tablets were coated using a Glatt GB2 pan coating machine, stored in controlled conditions, and tested for friability, moisture absorption, dissolution, and photostability.
Overall, the study indicates that while TiO2's role in light protection is critical for photosensitive drugs, alternative coatings may be feasible for drugs not requiring such protection.
Comments
No comments posted yet.