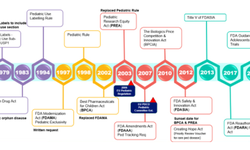
Optimizing pediatric product development for commercialization success
Ensuring medication adherence in children is challenging due to their changing physiology from infancy to adolescence, which complicates drug formulation. Pediatric formulations must offer flexible, accurate dosing, be easy to swallow, and taste acceptable. Multiparticulate systems (like granules, minitabs, or beads) are especially effective, allowing tailored dosing and administration via various formats (e.g., ODTs, sprinkles, suspensions).
This presentation explores the importance of pediatric-specific drug development, regulatory requirements, and formulation strategies—highlighting multiparticulates as a versatile solution.
Key learning objectives:
- Understand the importance of pediatric populations and the related regulatory landscape
- Identify age-specific dosing needs to improve acceptance and adherence
- Explore multiparticulates as an adaptable platform for pediatric formulations

Comments
No comments posted yet.