Scientific papers
Context: Sustained-release mini-tablets hold promise for pediatric drug delivery and as multiparticulate dosage forms.
Objective: Assess the feasibility of developing lipophilic matrix mini-tablets and examine the impact of Compritol® 888 ATO concentration on drug release from mini-tablets of different sizes prepared through direct compression.
Methods: A formulation containing theophylline as a model soluble drug, 15% w/w Compritol® 888 ATO as the inert matrix-forming agent, and dibasic dicalcium phosphate anhydrous and lactose as diluents was examined. This formulation was used to produce 12 mm tablets at various compression speeds and forces. The same formulation, along with additional formulations containing 25, 35, or 45% w/w Compritol® 888 ATO, was employed to produce 2, 3, and 4 mm mini-tablets.
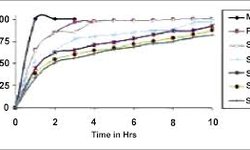
Results and Discussion: Sustained drug release from matrix tablets was observed over 12 hours, with the release rate influenced by compression force and speed. Matrix mini-tablets exhibited a faster drug release, and an increase in Compritol® 888 ATO concentration led to slower release rates. The drug release rate from matrix mini-tablets was inversely proportional to mini-tablet size (2 mm > 3 mm > 4 mm). Both matrix tablets and mini-tablets followed square-root of time kinetics.
Conclusion: Tailoring drug release from matrix mini-tablets can be achieved by adjusting mini-tablet size or the level of Compritol® 888 ATO in the formulation, offering potential applications in the development of pediatric formulations or multiparticulate dosage forms.
Comments
No comments posted yet.
Add a comment