Overcoming manufacturing challenges for accelerated drug development
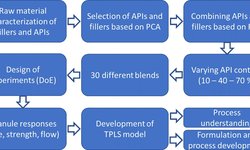
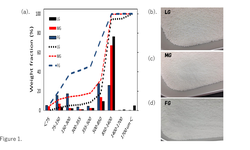
The ISPE has released a new Good Practice Guide focused on the development of control strategies for the continuous manufacturing (CM) of oral solid dosage (OSD) forms, including tablets. This guide underscores the benefits of continuous manufacturing over traditional batch processes: faster development and scale-up, enhanced process robustness, better quality assurance, and increased operational efficiency.
A key highlight is the chapter on continuous tableting, led by subject-matter experts such as Anthony Tantuccio, Fellow Scientist of Continuous Tableting at Hovione LLC. The guide provides a practical framework combining scientific, regulatory, and industry best-practice perspectives, specifically designed to help companies adopt continuous manufacturing of tablets and other solid dosage forms.
It covers essential aspects such as lifecycle management, control strategy design, equipment-process alignment, material-attribute understanding, and methods to build confidence in continuous tableting operations.
For professionals in tableting technology, the takeaway is that this guide signals a strong industry shift toward modernizing tablet manufacturing through continuous manufacturing and robust process control — marking an important step forward in the evolution of oral solid dosage production.

Comments
No comments posted yet.