Application bulletins
The success of a wet granulation process depends
on use of an excipient with excellent binding and
compaction properties. Mannitol is frequently used for
this purpose in solid dosage formulations due to its
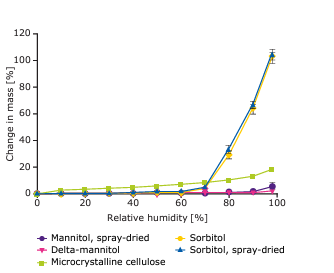
physicochemical properties; it is chemically inert, has
good compactibility and low hygroscopicity.1
While mannitols in general are increasingly used in
pharmaceutical formulations, the Parteck® mannitol
family of excipients offers important advantages.
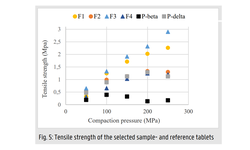
As shown in these studies, standard crystallized
mannitol in β-polymorphic form often lacks sufficient
binding and tableting properties for use with wet
granulation. In contrast, Parteck® Delta M, the
only commercially available mannitol excipient in δ
polymorph crystals, offers the inertness of mannitol
with excellent binding properties, enabling formulators
to more easily develop challenging formulations by
wet granulation. When fluidized bed granulation is
used or granulation with organic solvents is necessary,
Parteck® M 200 excipient is an excellent alternative as
it offers the surface area required to deliver the desired
compaction and flow properties.
Comments
No comments posted yet.
Add a comment