Scientific papers
Excipients play a crucial role in developing advanced, reliable formulations with enhanced patient compliance, optimizing plasma concentrations, and minimizing side effects. Modern excipients are expected to exhibit multifunctionality to enable unique applications not achievable with traditional excipients. Co-processing excipients is a strategy to achieve multifunctionality, wherein undesirable properties are masked, favorable attributes are retained, and new properties complement the substance.
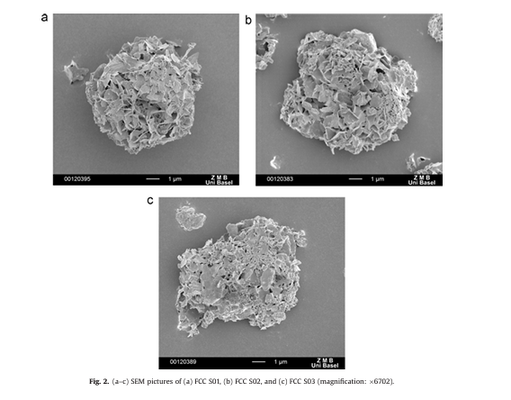
This thesis aimed to explore the applicability of a co-processed novel excipient, Functionalized Calcium Carbonate (FCC), within the realm of pharmaceutical technology. Mechanistic studies demonstrated that FCC-based tablet formulations exhibited mechanical properties equal to or superior to conventional excipients like microcrystalline cellulose (MCC), mannitol, or calcium carbonate. FCC tablets, possessing high tensile strength and porosity, were achieved even at low compressive pressures, attributed to the lamellar structure forming an intraparticle porous meshwork, providing a high specific surface area for particle bonding.
To address limitations such as poor flowability and high bulk density during direct compression, granulation proved effective. FCC granules prepared by roller compaction displayed excellent flowability and reduced bulk volume while preserving outstanding properties like compactability and compressibility. Dry granulation, particularly through roller compaction, emerged as the preferred process for preserving FCC's porosity and high surface area during scale-up on high-throughput tablet presses.
The thesis also investigated the applicability of FCC in the production of orally disintegrating tablets (ODTs). FCC, with its lamellar structure, addressed challenges related to insufficient hardness in the production of highly porous ODTs through direct compression. This discovery has the potential to revolutionize ODT production, leading to innovative possibilities.
To safeguard these valuable findings, a patent was filed for the production of ODTs using FCC. The comprehensive characterization of co-processed FCC showcased its potential as a versatile pharmaceutical excipient. FCC appears particularly promising for formulations requiring high porosity, high tensile strength, or both, including ODTs, carriers, adsorbents, floating tablets, effervescent tablets, controlled-release formulations, ultra-hard tablets (UHT), and cushioning agents.
Comments
No comments posted yet.
Add a comment















