Scientific papers
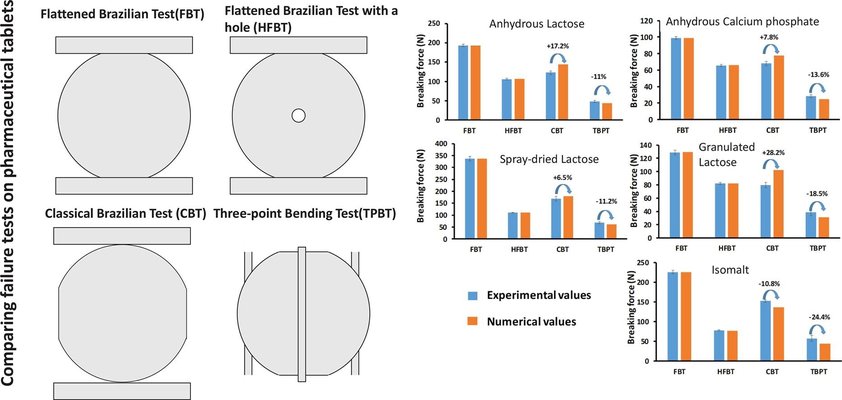
The mechanical strength is an important quality attribute of pharmaceutical tablets. It can be determined using different failure tests like the Brazilian test or the three-point bending test. Nevertheless, literature shows that different failure tests often give conflicting values of tensile strengths (TS), which are generally calculated using the maximum stress criterion as a failure criterion. This work started from the hypothesis that these discrepancies are in fact due to the application of this criterion which is not suited to study pharmaceutical tablets, first due to heterogeneity of the stress distributions during the tests and second due to the quasi-brittle nature of pharmaceutical tablets. As an alternative, a numerical fracture criterion which is known to be well-suited for quasi-brittle solids (cohesive zone model, CZM) was used and calibrated using experiments. Using this approach, the breaking forces obtained numerically were shown to be in fair agreement with the experimental ones. Above all, the numerical results made it possible to catch the trends when comparing the different failure tests one to another. Especially, the model made it possible to retrieve the factor 2 between the TS obtained by three-point bending and by diametral compression found in the literature.
Comments
No comments posted yet.
Add a comment