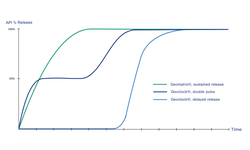
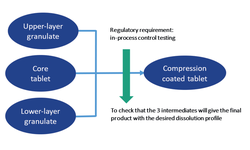
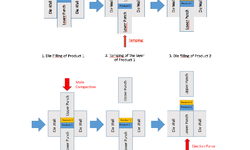
Scientific papers
Among the various pharmaceutical forms, tablets provide numerous advantages, including ease of administration, cost-effectiveness in production, and enhanced stability of biomolecules. Additionally, tablets facilitate alternative routes for local delivery of biopharmaceuticals, such as oral or vaginal administration, thus broadening their therapeutic applications and addressing the limitations associated with parenteral administration. However, there is currently limited information on the feasibility of developing biomolecules in tablet form.
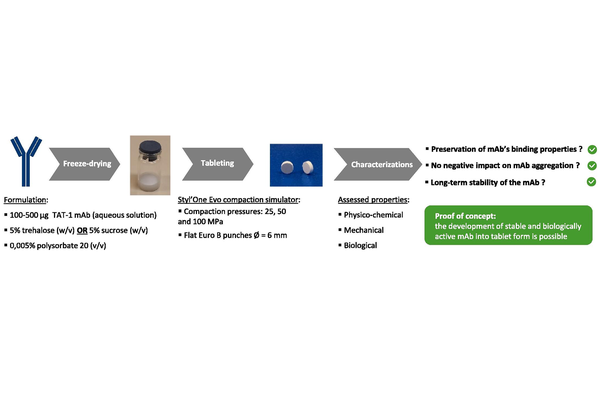
In this study, we evaluated the feasibility of developing monoclonal antibodies in tablet form while preserving their biological properties. We examined different excipients and process parameters to assess their impact on the antibody’s integrity during tableting. ELISA results indicate that applying compression pressure up to 100 MPa does not harm the antibody’s binding properties when formulated from a lyophilized powder with trehalose or sucrose as the primary excipient. This finding was corroborated by SPR and ultracentrifugation experiments, which demonstrated that neither the binding affinity for both Fc and Fab antibody fragments nor its aggregation rate is affected by the tableting process. Post-compression, the antibody-containing tablets were shown to be stable for six months at room temperature.
Comments
No comments posted yet.
Add a comment