Scientific papers
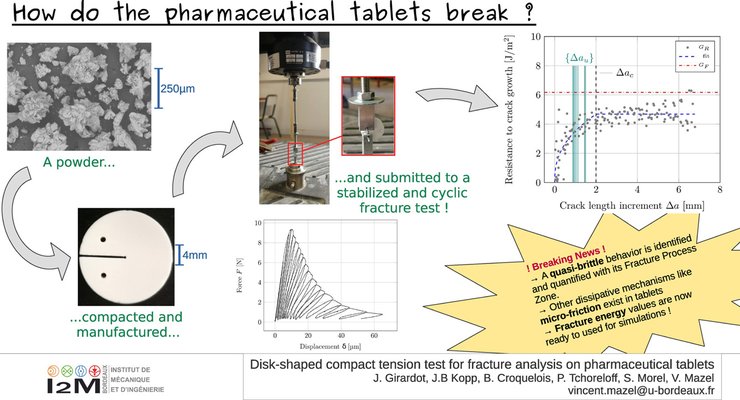
Pharmaceutical tablets must fulfill various requirements, with mechanical strength playing a pivotal role. While the diametral compression test (DCT) is typically employed for evaluation, it can yield unstable failures. By subjecting tablets to load–unload cycles during a DCT, it has been demonstrated that the concept of equivalent linear elastic fracture mechanics, usually applicable to Mode I fracture behavior, can be successfully employed. In this context, the equivalent elastic crack growth resistance, commonly referred to as the resistance curve (R-curve), was determined for the studied material. This analysis unveiled the emergence of a Fracture Process Zone (FPZ), indicative of a quasi-brittle behavior. Furthermore, the cyclic loading applied during the fracture test revealed the presence of a second dissipative mechanism leading to residual crack opening, primarily attributed to friction phenomena within the FPZ.
Comments
No comments posted yet.
Add a comment