Scientific papers
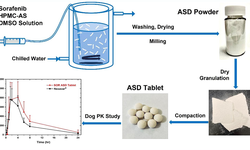
The objective was to improve the dissolution of lornoxicam (LOR) and create mini-tablets with an optimized system to achieve a fast-release multi-particulate formulation. LOR systems were prepared through co-evaporation with either polyethylene glycol 6000 or Pluronic® F-68 (PLU) and adsorption onto Neusilin® US2 alone or co-adsorption in the presence of varying amounts of polysorbate 80. All systems underwent characterization using FT-IR, differential scanning calorimetry, X-ray diffraction, flowability, and dissolution techniques. Mini-tablets were then prepared using the system with the optimal dissolution profile and flowability. The mini-tablets' tensile strengths, content uniformity, and dissolution profiles were assessed, and the effects of different excipients and storage conditions on mini-tablet properties were investigated. The optimized rapid-release LOR mini-tablets were further examined for their in vivo pharmacokinetic profile. The co-evaporate of LOR with PLU exhibited significantly faster dissolution and superior flowability. This system, along with three directly compressible excipients (Cellactose® 80, StarLac® (STA), and Emcompress®), was evaluated for mini-tablet formulation. The formulation with STA yielded optimal results in terms of tensile strength, content uniformity, and rapid drug release during a 3-month stability study, and was chosen for further in vivo assessment. The pharmacokinetic profile demonstrated the potential of the mini-tablets to achieve rapid release and enhanced absorption of LOR.
Comments
No comments posted yet.
Add a comment