Scientific papers
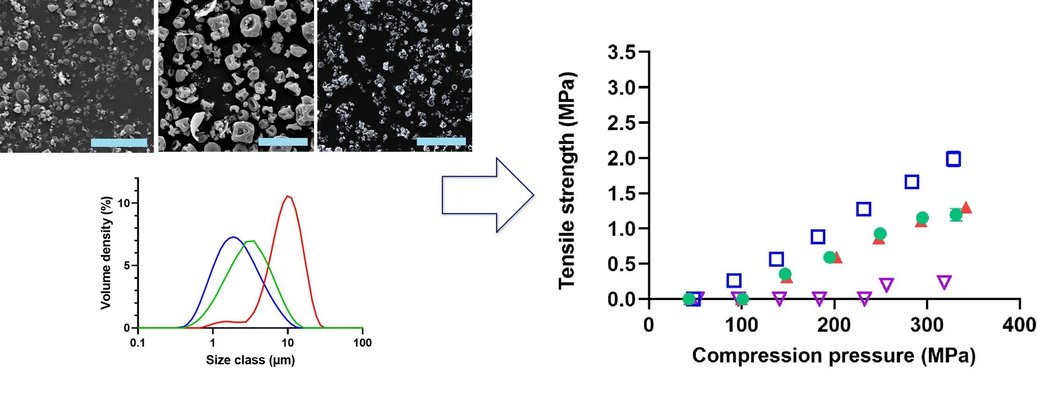
The oral administration of proteins and peptides is an appealing option for dosing due to its high patient compliance. However, formulating these macromolecules for oral delivery often involves incorporating permeation enhancers, which are typically poorly compactable. This can present challenges in the compression behavior during tablet manufacturing processes. This study demonstrates that addressing poor compression behavior is achievable by customizing the properties of peptide or protein particles, particularly in high-dose tablet formulations. Spray-dried particles with diverse particle sizes and morphologies were generated and thoroughly characterized. The tabletability of these particles was then assessed in formulations with varying compressibility characteristics. The results indicated that the most significant improvement in tabletability occurred with small, non-hollow spray-dried insulin particles in both well-tabletable and poorly tabletable formulations. Interestingly, the enhancement was more pronounced in the poorly tabletable formulation than in the well-tabletable one. This underscores the critical role of the particle properties of the active pharmaceutical ingredient (API) in evaluating the manufacturability of formulations with poor tableting characteristics.
Comments
No comments posted yet.
Add a comment