Scientific papers
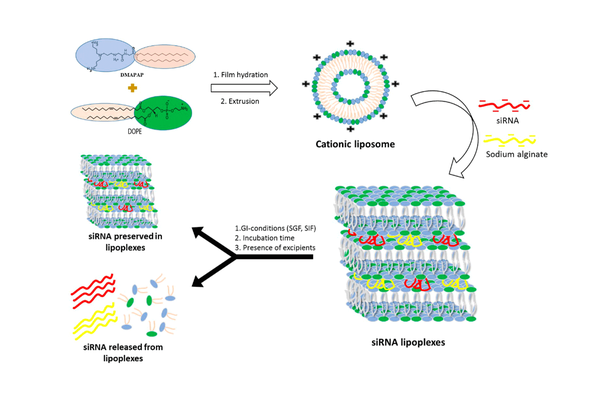
It is essential to incorporate siRNA into nanocarriers to facilitate its intracellular delivery, as siRNA alone cannot penetrate cells. However, the incorporation of these nanocarriers into oral solid dosage forms and their behavior in the gastrointestinal environment have not been thoroughly investigated. In this study, the destiny of (i) naked siRNA, (ii) freshly prepared siRNA lipoplexes, and (iii) tableted siRNA lipoplexes in simulated gastric and intestinal fluids was examined. The siRNA, whether released from or protected within the lipoplexes, was quantified using gel electrophoresis, and siRNA efficacy was evaluated in cell transfection.
The freshly prepared lipoplexes maintained their siRNA load and transfection efficiency completely intact during 1 hour of incubation in simulated gastric fluid at 37 °C. However, in simulated intestinal fluid, although there was no release of siRNA from lipoplexes after 6 hours of incubation, gene silencing efficacy significantly decreased even after 1 hour of exposure. Lipoplexes obtained from tablets effectively shielded siRNA in simulated gastric fluid, thereby preserving gene silencing efficacy. On the contrary, their incubation in simulated intestinal fluid resulted in substantial siRNA release and decreased gene silencing efficacy. These findings offer a comprehensive understanding of the fate of siRNA under gastrointestinal conditions, whether simply loaded in lipoplexes or formulated in tablet form.
Comments
No comments posted yet.
Add a comment