Scientific papers
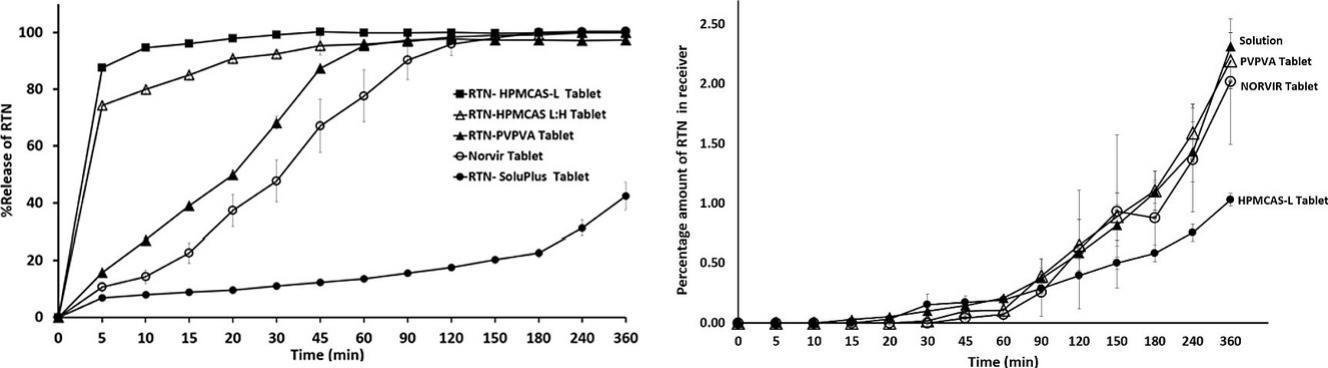
Tablets remain the most widely used oral dosage form, valued for their rapid production, cost efficiency, stability, and patient adherence. Amorphous solid dispersions (ASDs) are a proven strategy to enhance the solubility and bioavailability of poorly soluble drugs; however, systematic approaches for transforming ASD powders into robust tablet formulations are limited. This work focused on optimizing the formulation and manufacturing process for ritonavir–HPMCAS-L ASD tablets and benchmarking their dissolution and permeation behavior against PVP-VA–based tablets. Microcrystalline cellulose (MCC) and dibasic calcium phosphate (DCP) were assessed as filler excipients, while croscarmellose sodium (CCS), crospovidone (CP), and sodium starch glycolate (SSG) were tested as disintegrants. The study investigated two ASD powder loadings, two disintegrant concentrations, and two spray-drying inlet temperatures (70 °C and 140 °C). Process optimization also compared direct compression with dry granulation under varying compaction forces. The optimal formulation for ritonavir–HPMCAS-L ASD tablets, showing strong in vitro dissolution performance, consisted of dry granulation (14 kN pre-compression + 7 kN main compression), 65.5% ASD intermediate, 10% CCS, 24% MCC, and 0.5% magnesium stearate. These optimized conditions were applied to tablets prepared with PVP-VA, SoluPlus, HPMCAS-L, and HPMCAS-L:H. Both PVP-VA and HPMCAS-L tablets achieved favorable in vitro dissolution, but permeation testing revealed that PVP-VA tablets provided higher ritonavir transport compared with HPMCAS-L. Moreover, PVP-VA tablets demonstrated permeation profiles similar to commercial Norvir tablets and Norvir powder solution. These findings suggest that colloidal species formed during dissolution may play a critical role in drug absorption from ASD formulations, potentially beyond the benefits of dissolution alone.
Comments
No comments posted yet.
Add a comment