Scientific papers
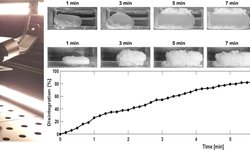
This study aimed to explore the granule properties and processing conditions leading to capping and lamination phenomena in a high drug load, wet granulated, film-coated product. A comprehensive analysis was conducted by integrating historical industrial data with parameters obtained from compaction simulator testing of representative samples. Employing a systematic approach based on batch statistical modeling, the goal was to discern patterns among batches associated with variations in input variables.
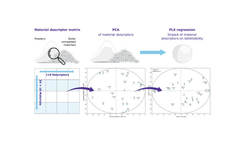
The modeling of batch evolution unveiled the typical process fingerprint and expected variability, establishing that defects were not correlated with process evolution excursions. Utilizing class-based modeling and ANOVA facilitated the identification of statistically significant differences between input variables, specifically active ingredient particle size and granulation water amount. This approach enabled adjustments to be made for process improvement.
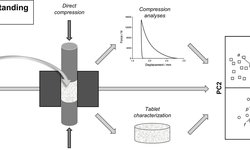
The model developed with parameters from the compaction simulator not only validated inter-batch differences but also suggested the mechanism behind capping/lamination, indicating a higher elastic recovery rate. Strain rate sensitivity (SRS) values indicated the product's sensitivity to certain processing conditions, including dies wear. The negative correlation between SRS and bulk density provided a basis for predicting suitable tableting conditions to enhance tablet hardness.
Comments
No comments posted yet.
Add a comment