Scientific papers
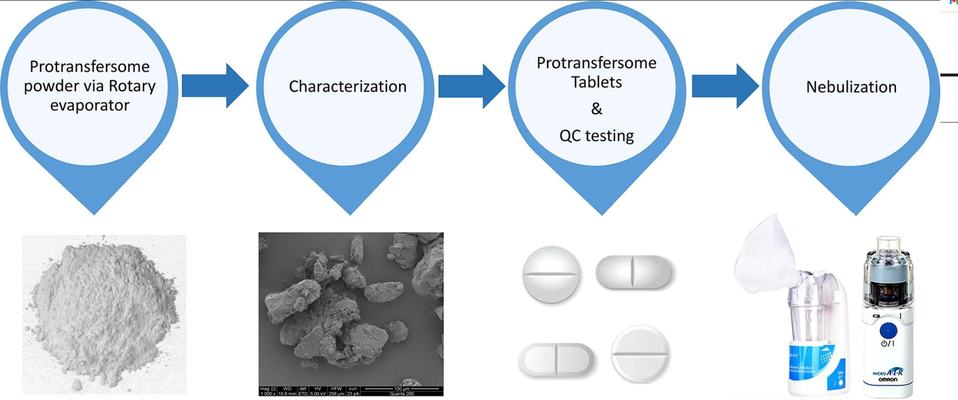
A straightforward approach was employed to develop innovative paclitaxel (PTX) loaded protransfersome tablet formulations intended for pulmonary drug delivery. Leveraging the expansive surface area of the pulmonary system, the goal was to achieve localized anti-cancer drug deposition within the lungs. Protransfersomes, initially in dry powder form, transform into liquid dispersions containing vesicles upon hydration. The protransfersome powder formulations (F1–F27), referred to as Micro formulations based on transfersome vesicle size post-hydration, were crafted using a phospholipid (Soya phosphatidylcholine (SPC)), three distinct carbohydrate carriers (Lactose monohydrate, LMH; Microcrystalline cellulose, MCC; and Starch), and three surfactants (Span 80, Span 20, and Tween 80) in various lipid phase to carrier ratios (1:05, 1:15, and 1:25 w/w). Paclitaxel (PTX) was incorporated as the model drug. The hydrophobic chain of SPC aimed to enhance PTX solubility, entrapment, and targeted delivery via transfersome vesicles.
Out of the 27 Micro protransfersome formulations, PTX-loaded LMH powder formulations F3, F6, and F9 (with a 1:25 w/w lipid phase to carrier ratio) exhibited excellent powder flowability, as evidenced by the angle of repose (AOR), and a good compressibility index due to the associated smaller and uniform particle size and shape of LMH. Following hydration, these formulations also displayed smaller volume median diameters (VMD) ranging from 5.65 ± 0.85 to 6.76 ± 0.61 µm and PTX entrapment of 93–96%. The hydrated transfersome formulations (F3, F6, and F9) were converted into Nano size through probe sonication, referred to as Nano formulations. These Nano formulations were further transformed into dry powder via spray drying (SD) (F3NSD, F6NSD, and F9NSD) or freeze drying (FD) (F3NFD, F6NFD, and F9NFD).
After the manufacture of protransfersome tablets (i.e., nine formulations), quality control tests were conducted in accordance with the British Pharmacopeia (BP). Only the Micro formulations protransfersome tablets (F3, F6, and F9) passed the uniformity of weight test, exhibited high crushing strength, and maintained tablet thickness compared to SD or FD protransfersome tablets. The Micro protransfersome formulations (F3, F6, and F9) converted into tablets demonstrated a shorter nebulization time and high output rate when using an Ultrasonic nebulizer compared to a Vibrating mesh nebulizer (Omron NE U22). Based on formulations, characterizations, and nebulizer performance, the Micro protransfersome tablet formulations F3, F6, and F9 (with a 1:25 w/w ratio) and Ultrasonic nebulizer were found to be a superior combination, exhibiting enhanced output efficiency. Moreover, PTX-loaded F3, F6, and F9 tablet formulations (10%) exhibited toxicity (60%, 68%, and 67% cell viability) to cancer MRC-5 SV2 (immortalized human lung cells) while remaining safe for MRC-5 (normal lung fibroblast cells) cell lines.
Comments
No comments posted yet.
Add a comment















