Scientific papers
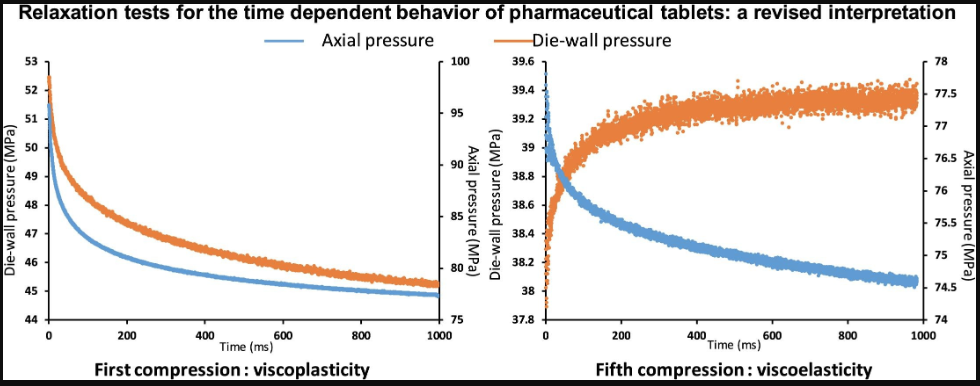
Relaxation tests are commonly used in the pharmaceutical industry to evaluate the strain rate sensitivity of powders and tablets. In these tests, a constant strain is applied to the powder within a die, and the resulting stress is tracked over time. Interpreting these results is challenging due to the simultaneous occurrence of two key physical phenomena: viscoelasticity and viscoplasticity. Both cause a decrease in axial pressure, making it difficult to distinguish between them based on axial pressure alone. This study demonstrates that monitoring die-wall pressure during relaxation can help differentiate between these phenomena. Theoretical analysis showed that while viscoplasticity leads to a decrease in die-wall pressure, an increase in die-wall pressure indicates viscoelastic relaxation. This was experimentally confirmed using specialized compaction cycles on four pharmaceutical excipients. The results revealed that viscoplasticity dominates at low pressures, whereas viscoelasticity becomes more significant at high pressures. These findings suggest that at low pressures, relaxation tests can effectively assess viscoplastic properties. However, high pressures should be avoided, as viscoelastic effects may become prominent, complicating interpretation due to the interaction of both phenomena.
Comments
No comments posted yet.
Add a comment













