Scientific papers
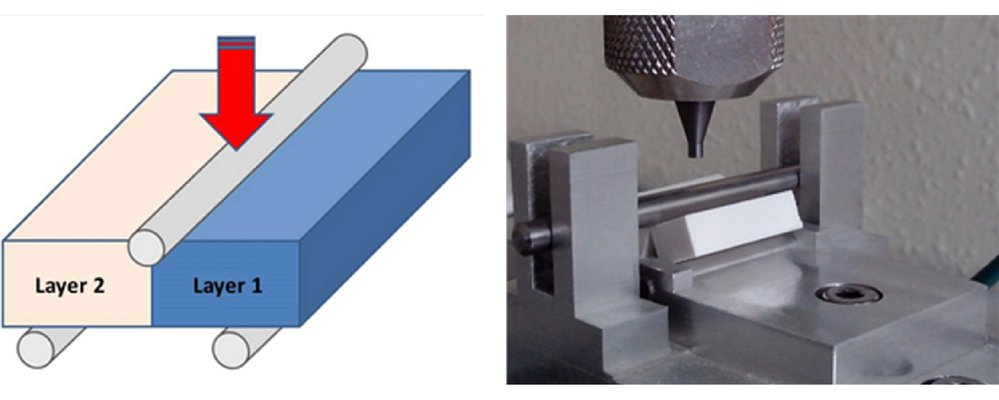
The impact of the elasticity of various pharmaceutical materials on the interfacial adhesion in bilayer tablets was explored. The elastic properties of five pharmaceutical products were assessed based on their total elastic recovery. To evaluate the interfacial strength of the bilayer tablets, a novel flexural test was introduced. This test configuration allowed the experimental breaking force to be directly associated with the strength of the interfacial layer. Depending on the materials involved, the fracture either occurred over the interface or within one of the two layers. In most instances, the highest breaking forces were achieved when the materials exhibited similar elastic recovery. Conversely, when materials had different elastic recovery, the breaking forces were reduced. Statistical analysis was applied to examine the observed variations in interfacial mechanical strength. Such an approach holds significance in the context of the growing emphasis on the Quality by Design (QbD) concept in the pharmaceutical industry.
Comments
No comments posted yet.
Add a comment