Scientific papers
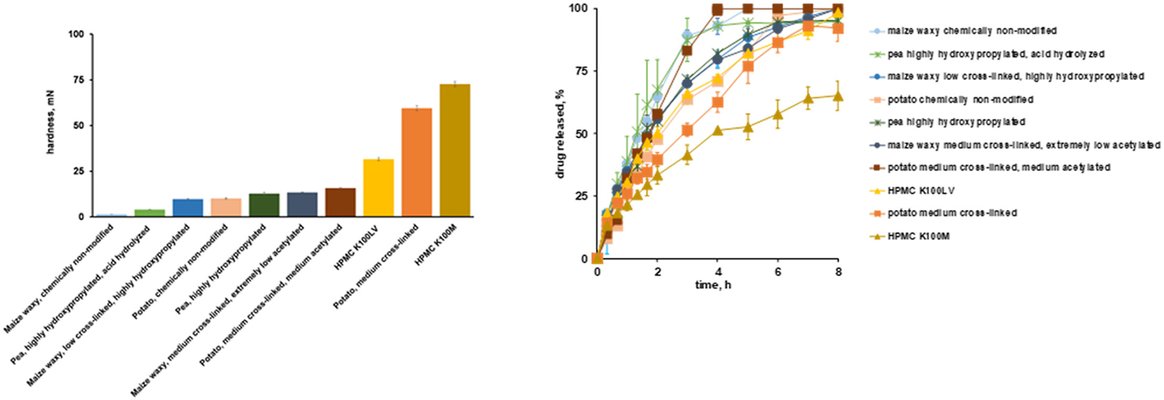
Various starch types were employed in the formulation of controlled release tablets containing diprophylline through direct compression. The study explored the influence of starch's natural origin, potential chemical modifications (such as cross-linking with phosphate or adipate, hydroxypropylation, acetylation to varying degrees, partial hydrolysis), and the type of pre-gelatinization process (lab scale with ethanol and oven drying versus industrial scale drum drying) on the resulting kinetics of drug release. To enhance comprehension, texture analysis of hydrogels formed upon exposure to the release medium, along with optical and scanning electron microscopy and X-ray powder diffraction measurements, was conducted. Additionally, a "quick test" employing a texture analyzer was introduced to rapidly assess a specific starch type's capacity to control the ensuing drug release rate. Two hydroxypropyl methylcellulose types (HPMC K100M and K100 LV) were included for comparative purposes. Notably, the "quick test" effectively identified differences in the mechanical strength of hydrogels formed upon contact with aqueous fluids, aligning well with observed drug release patterns measured using a USP III ("Bio’-Dis") apparatus at 30 rpm. However, diprophylline release showed minimal impact with the investigated starch types when using a USP basket apparatus at 75 rpm, attributed to the significantly lower mechanical stress experienced by the hydrogels under these conditions. Furthermore, caution is advised when studying starch types pre-gelatinized at the lab scale using ethanol precipitation and oven drying, as the obtained starch granules may exhibit substantially different key properties compared to those obtained through industrial scale drum drying, resulting in distinct drug release patterns.
Comments
No comments posted yet.
Add a comment















