Scientific papers
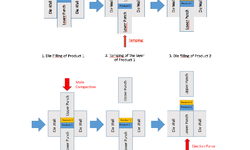
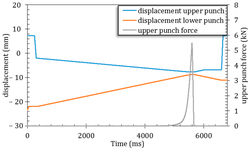
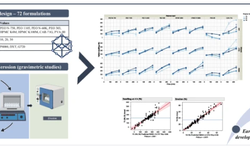
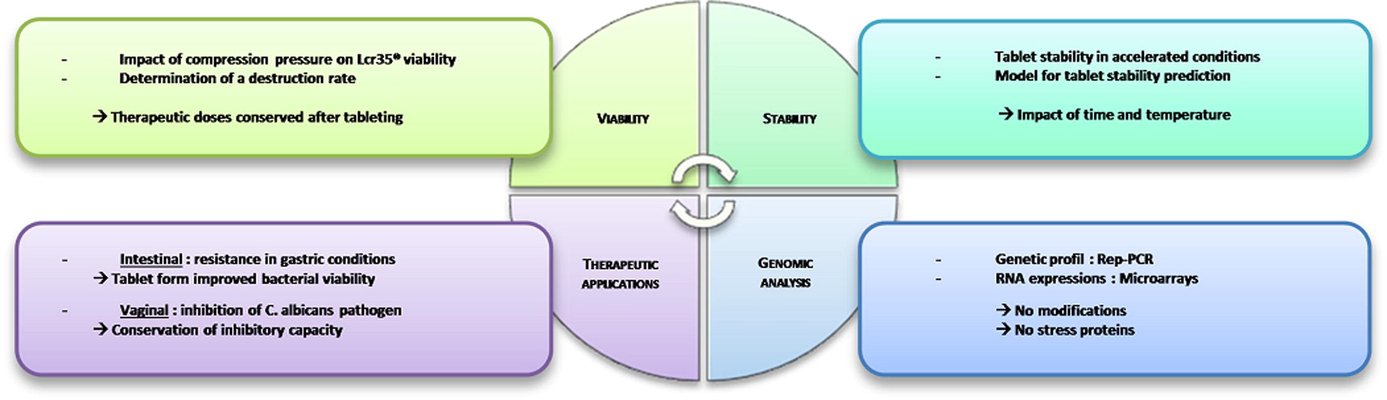
The acknowledged positive impacts of probiotic bacteria on human health have sparked increasing interest in pharmaceutical research and development. However, for these bacteria to remain viable upon reaching their target, they must withstand the manufacturing process and biological pathway. Tablets are considered an optimal form for protecting acid-labile drugs, yet the tableting process could impose additional stress on the bacteria. This study assessed the initial impact of compression pressure on the Lcr35® strain in both a vaginal (Lcr regenerans®) and an intestinal (Lcr restituo®) formulation. A stability study on the tablets revealed their beneficial effect. Destruction rates (k) indicated that bacterial stability was higher in tablets compared to powders (k_powders > k_tablets). A novel mathematical model was developed, integrating compression and temperature parameters to predict bacterial viability at any pressure and time. Furthermore, the genetic profile of Lcr35® (Rep-PCR, microarrays), its acidity resistance, and its ability to inhibit Candida albicans growth post-compression were evaluated to establish the Target Product Profile (TPP) in a Quality by Design (QbD) approach. Rep-PCR validated strain identity, microarrays demonstrated genetic stability post-compaction, the ability to inhibit C. albicans growth was maintained, and resistance to gastric conditions was even improved by tableting. Tablets as a dosage form ensure the delivery of an adequate bacterial amount to the therapeutic target (intestinal or vaginal) and maintain product stability until consumption.
Comments
No comments posted yet.
Add a comment