Scientific papers
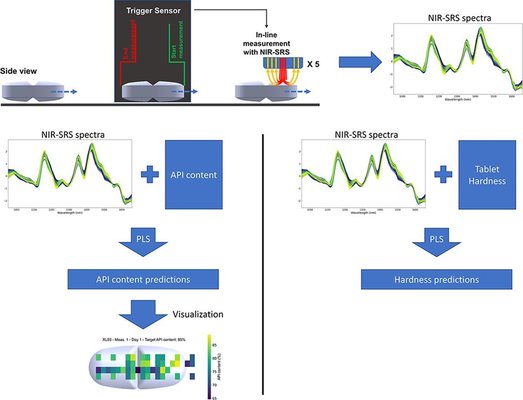
To achieve real-time release of tablets, it is imperative to monitor and control quality attributes using Process Analytical Technology tools like near-infrared spectroscopy (NIRS). The authors investigated the applicability of NIR-Spatially Resolved Spectroscopy (NIR-SRS) for continuous real-time monitoring and control of content uniformity, hardness, and homogeneity in tablets with unconventional dimensions. A user-friendly research and development inspection unit, serving as standalone equipment, was employed for analyzing small oblong tablets with deep-cut break lines. A total of 66 tablets, varying in hardness and Active Pharmaceutical Ingredient (API) content, underwent inspection, with each tablet analyzed five times and measurements repeated on three different days. Partial Least Squares (PLS) models were developed to assess content uniformity and hardness, with the former exhibiting higher accuracy.
The authors endeavored to visualize tablet homogeneity through NIR-SRS spectra by regressing all spectra obtained during a single measurement using a content uniformity PLS model. The NIR-SRS probe showcased its potential for real-time release testing by swiftly monitoring content uniformity, hardness, and visualizing homogeneity, even for tablets with challenging dimensions.
Comments
No comments posted yet.
Add a comment