Scientific papers
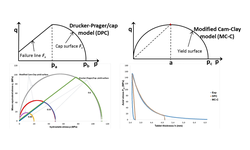
The pharmaceutical powder compression cycle involves multiple stages, each associated with intricate mechanisms such as particle rearrangement, densification, inter-particle interaction, friction, elastic and plastic deformation, and particle fragmentation. A comprehensive understanding of these processes is crucial for gaining insights into tabletting and addressing potential issues. This study focuses on evaluating the capping problem through computer modeling of the pharmaceutical powder compression and consolidation process, employing the finite element analysis (FEA) method with the Drucker–Prager Cap (DPC) model. The simulation studies specifically targeted the widely used pharmaceutical excipient, Avicel PH 102.
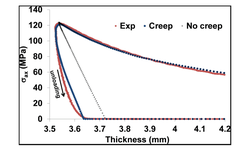
The computer simulation revealed that when the friction coefficient (μ) exceeds 0.2, the elastic recovery of the powder bed increases, leading to excessive dilation during decompression and the potential for capping issues during tabletting. Additionally, the study observed that the powder's lubrication level and tooling geometry influenced the pressure distribution throughout the powder bed. Inhomogeneity in stress and density distribution may result in inadequate tablet consolidation and damage during the decompression stage. The simulation study suggests that the optimal friction coefficient value for Avicel PH 102 under specific compaction parameters (e.g., punch size, R-value of CF tablet, etc.) is μ = 0.15. It's important to note that this study did not assess the variation of DCP parameters due to relative density changes during the compression process.
In summary, this simulation study demonstrates the successful application of finite element analysis tools to address capping issues associated with a particular pharmaceutical formulation. Such an approach has the potential to reduce the need for an extensive amount of analysis work during the preformulation stages, offering a more efficient means of optimizing formulations.
Comments
No comments posted yet.
Add a comment