Scientific papers
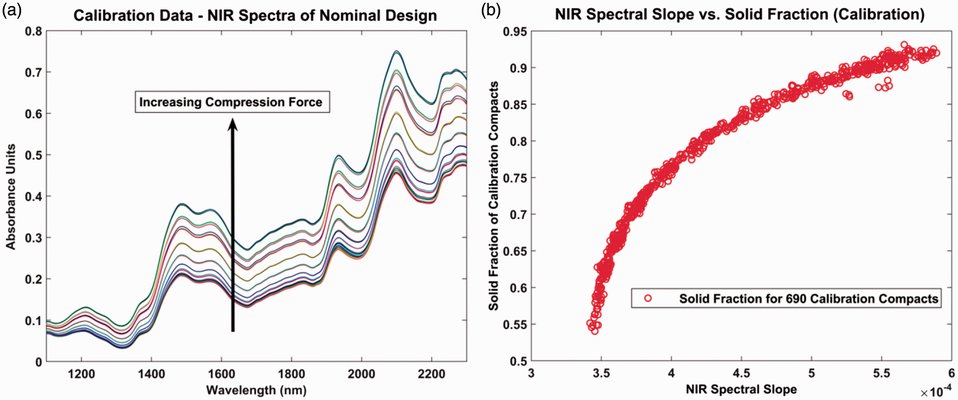
The objective of this study is to develop a near-infrared (NIR) method for predicting the solid fraction (SF) of dry granulated ribbons with formulation variability. The research explores the influence of unmodeled chemical variability and different regression approaches on the method's performance. An excipient-only formulation system was employed in the study. Calibration compacts were generated with both chemical and processing variability, and NIR spectra were then collected. Partial least squares (PLS) and spectral slope algorithms were applied to model compact SF. Subsequently, these models were used to predict the SF of test ribbons and compacts containing an active pharmaceutical ingredient (API) at various concentrations. The risk associated with unmodeled chemical variation was evident through the emergence of new peaks and reduced baseline absorbance in the NIR spectra. The spectral slope method proved more effective in managing this risk, displaying higher robustness with increasing API concentrations. The diminished robustness of the PLS approach was linked to the impact of chemical variability on both spectral baseline and peak absorbance. The spectral slope approach demonstrated a prediction error of around 5% at a 10% drug load. Recognizing the risks tied to unmodeled variability can inform the development of NIR methods as a sparing technique for low-dose product development.
Comments
No comments posted yet.
Add a comment