Scientific papers
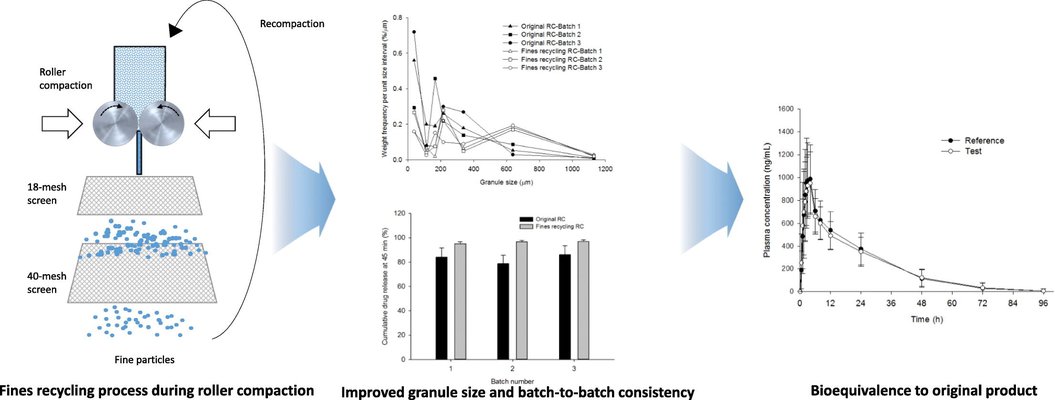
This study aims to enhance the manufacturing process of a generic immediate-release tablet containing erlotinib hydrochloride by introducing a fines recycling step during roller compaction. The initial powder mixture faced inconsistency in feeding into the roller compactor due to a substantial fraction of small-sized API particles, resulting in the production of poorly flowing granules with a high fines ratio. To address this issue, a fines recycling step was incorporated into the existing roller compaction process to mitigate the challenges associated with poor granule flow. Laboratory-scale roller compactor and tablet simulator setups were employed to generate granules under various process conditions. The impact of dry granulation parameters on size distribution, API distribution powder flow, compaction properties, and dissolution profile was systematically assessed. Following the fines recycling process, the granule batch exhibited significantly improved size distribution and flowability, while maintaining satisfactory tablet tensile strength and achieving a rapid dissolution profile. Implementing the fines recycling process at a commercial scale resulted in consistent dissolution performance and batch-to-batch uniformity, as evidenced by bioequivalence to the reference product. Understanding the influence of the fines recycling process on granule properties provides valuable insights for fine-tuning the dry granulation process to achieve optimal product quality.
Comments
No comments posted yet.
Add a comment













