Bilayer tablet manufacturing - An overview
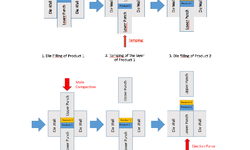
The text highlights the advantages and challenges of bilayer tablets in pharmaceutical formulations. Bilayer tablets offer controlled delivery of one or more active pharmaceutical ingredients (APIs) within a single tablet, enhancing patient compliance and simplifying medication regimens. Despite these benefits, their novel nature often requires additional formulation activity for commercialization, leading to technical challenges such as tablet delamination and cross-contamination. AbbVie addresses these challenges by investing in technology solutions and customizing equipment for efficient manufacturing. Optimal bilayer tablet formulation involves compressing two formulations into a single tablet while maintaining a physical barrier between them, enabling controlled release profiles. AbbVie's extensive manufacturing capabilities and quality compliance practices ensure safe and high-quality production. The company offers experience in bilayer manufacturing at its facilities in Cork, Ireland; Ludwigshafen, Germany; Lake County, Illinois; and Barceloneta, Puerto Rico, catering to clients' needs for bilayer tablet production.

Comments
No comments posted yet.