Scientific papers
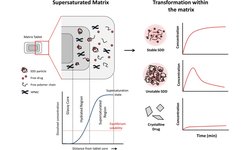
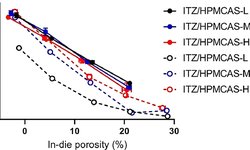
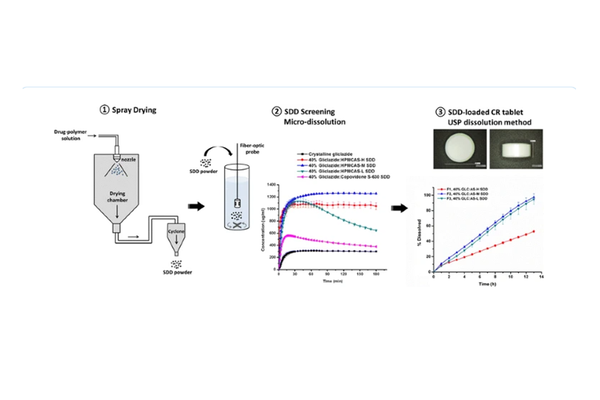
The objective of this study was to create a hydrophilic oral controlled release system (CRS) utilizing the amorphous form of gliclazide, a BCS class II compound listed in the WHO essential medicines. Spray-dried dispersions (SDDs) of gliclazide were generated under fully automated conditions using different grades of hydroxypropyl methylcellulose acetate succinate (HPMCAS) or copovidone as carriers. The solid-state properties of the prepared SDDs were characterized through X-ray powder diffraction (XRPD), scanning electron microscopy (SEM), modulated differential scanning calorimetry (MDSC), and Fourier-transform infrared spectroscopy (FTIR). Supersaturated micro-dissolution testing in fasted state-simulated intestinal fluid demonstrated prolonged supersaturation, with solubility increases ranging from 1.5- to 4.0-fold. The solubility and stability characteristics of the most favorable SDDs, based on relative dissolution area under the curves (AUCs) (AUC(SDD)/AUC(crystalline)) and stable supersaturated state concentration ratio up to 180 min (C180/Cmax), were determined. The optimized gliclazide-SDD amorphous forms were incorporated into matrix tablets with HPMC blends using a compaction simulator. The developed matrix systems underwent standard USP dissolution testing, resulting in linear dissolution profiles with varying slopes indicating different rates of dissolution. Six-month storage stability testing demonstrated that the dissolution profiles remained stable, with a "similarity factor" (f2 = 85). The results indicate that utilizing various HPMCAS as a drug carrier in the spray-drying process yields homogeneous single-phase SDDs that are stable and hold promise for inclusion into HPMC-based hydrophilic matrix systems.
Comments
No comments posted yet.
Add a comment