Scientific papers
High-molecular-weight hypromellose (HPMC) and hydroxypropyl cellulose (HPC) are widely recognized as extended-release polymers. While conventional high-molecular-weight HPMCs are preferred for extended-release applications, their use in twin-screw melt granulation is limited due to processing challenges at low temperatures and the risk of drug degradation at high temperatures. On the other hand, high-molecular-weight HPC (Klucel®) is suitable for melt granulation processes. This study aims to assess the processability and dissolution behavior of HPC GXF (Klucel® GXF) and a recently introduced type of hot-melt extrudable HPMC (Affinisol®) in extended-release metformin hydrochloride formulations using twin-screw melt granulation.
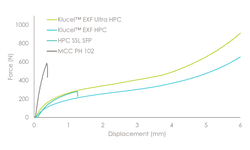
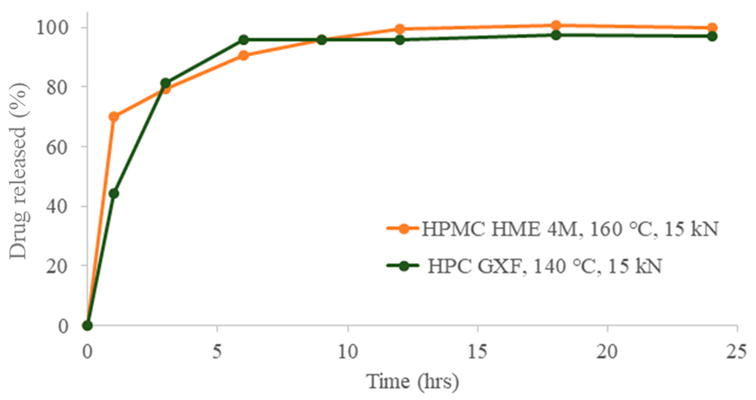
Powder blends, comprising 75% w/w metformin HCl and 25% w/w polymeric binder, were prepared and granulated at processing temperatures of 160, 140, 120, and 100 °C. HPMC HME 4M (Affinisol® 4M) yielded fine powder, indicating minimal granulation at temperatures below 160 °C, with resulting tablets exhibiting capping during compression. In contrast, tablets of acceptable quality were obtained with HPC GXF at all processing temperatures. Rheological studies, including capillary rheometry for steady shear rate viscosity and rotational rheometry for time and temperature superposition data, revealed that HPC GXF exhibited greater thermoplasticity than HPMC HME 4M, enabling granulation at low temperatures.
Tablets compressed with granules obtained at 160 °C with both binders showed comparable dissolution profiles. High-molecular-weight HPC GXF demonstrated superior processability at low temperatures and provided adequate tablet strength for the melt granulation of metformin HCl.
Comments
No comments posted yet.
Add a comment