Scientific papers
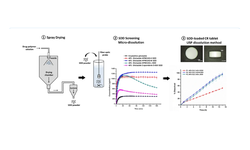
Apremilast, a selective PDE4 inhibitor approved for various inflammatory disorders, is categorized as a BCS-IV drug and exists in seven polymorphic forms. This study outlines the creation of a sustained-release (SR) drug delivery system based on an amorphous solid dispersion (ASD). The development process utilized a simplified, material-sparing ASD formulation approach to identify optimal carrier polymers for achieving the best drug loadings. Lead formulations were determined as HPMCAS-M at 20% and Copovidone at 40% drug loadings.
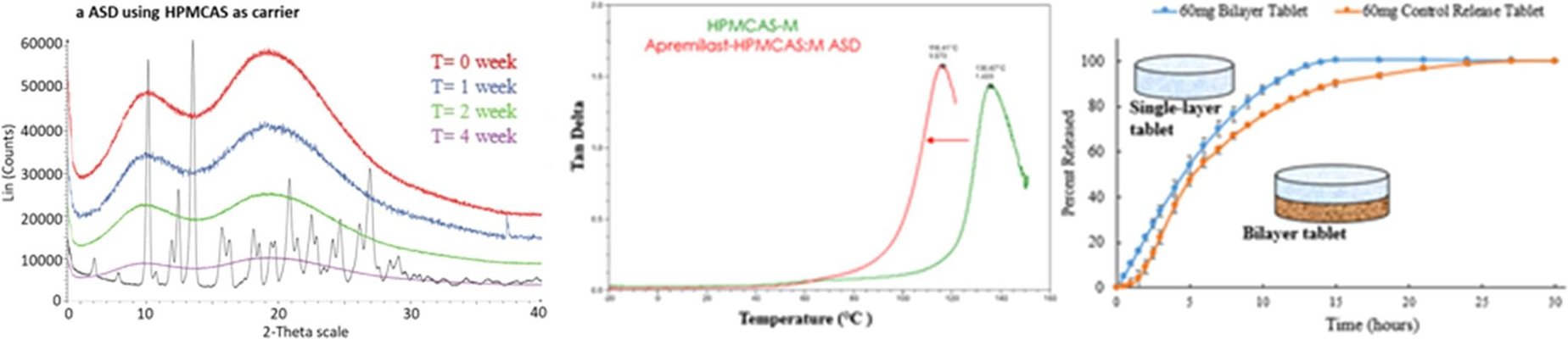
A stable single-phase amorphous system of apremilast was produced through spray drying and comprehensively characterized using mDSC, XRPD, DMA, micro-dissolution, dissolution, and accelerated stability analysis. The micro-dissolution study of the ASD confirmed the attainment and maintenance of a supersaturated state over 3 hours, showing an 8-fold increase in solubility compared to its crystalline counterpart. Monolithic and bilayer sustained-release HPMC tablet matrices containing 30 mg or 60 mg of the ASD system were successfully manufactured. During dissolution, these tablets exhibited gradual swelling, erosion, and disentanglement over 15–20 hours, achieving over 90% drug release.
The designed sustained-release amorphous matrix system demonstrated the ability to enhance apremilast solubility, dissolution rate, and prevent recrystallization or polymorphic interconversion by stabilizing its amorphous form. This innovative development holds promise for enabling once-daily drug administration, potentially improving both bioavailability and patient compliance.
Comments
No comments posted yet.
Add a comment