Scientific papers
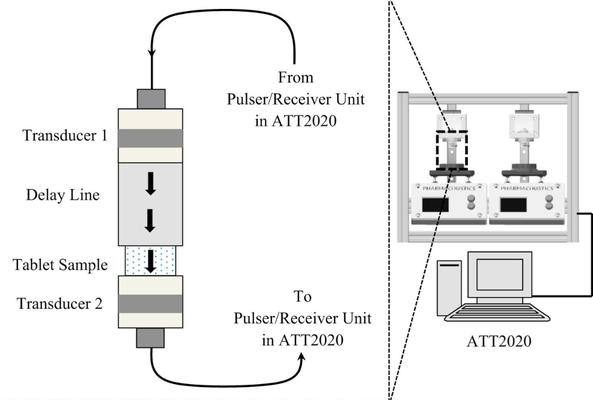
The detection of capping, a prevalent mechanical defect in tablet manufacturing, is crucial for pharmaceutical industry processes. This study introduces a non-destructive contact ultrasonic method for identifying capping risk in pharmaceutical compacts formed under different compression forces and speeds. The research demonstrates that the extracted mechanical properties serve as early indicators for potential capping, even before visible damage occurs. By analyzing X-ray cross-section images and an extensive dataset of waveform information, it is revealed that the mechanical properties and acoustic wave propagation characteristics undergo significant modulation due to internal cracks and capping at higher compaction speeds and pressures. Furthermore, the experimentally derived properties are correlated with directly measured porosity and tensile strength of compacts made from Pearlitol®, Anhydrous Mannitol, and LubriTose® Mannitol, produced at varying compaction speeds and pressures. The impact of compaction speed and pressure on the resulting compacts' porosity and tensile strength is quantified and associated with their acoustic characteristics and mechanical properties. This comprehensive experimental approach, along with the reported wave propagation data, holds potential applications in establishing manufacturing design spaces during development, predicting capping in continuous tablet manufacturing, and providing online monitoring of tablet mechanical integrity to reduce batch-to-batch variations in end-product quality.
Comments
No comments posted yet.
Add a comment