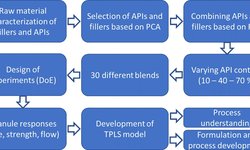
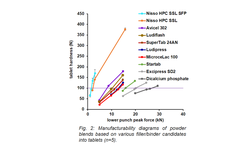
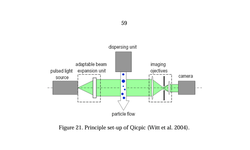
Scientific papers
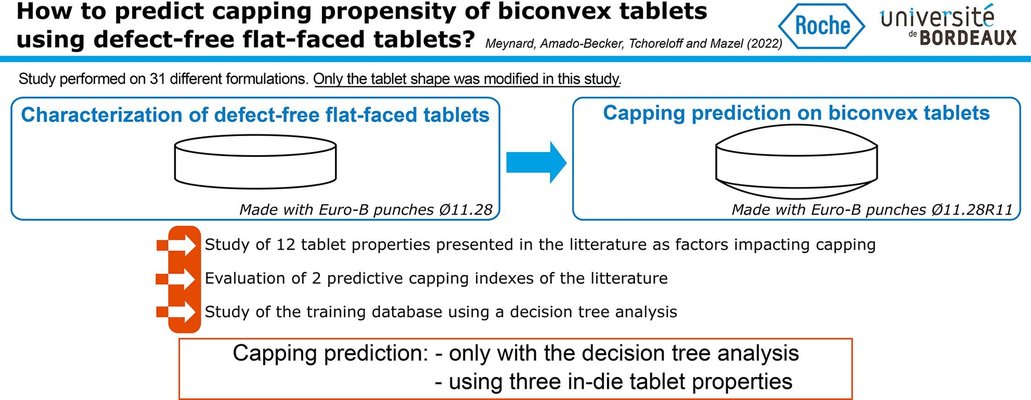
Anticipating tablet defects, particularly capping, during the pharmaceutical manufacturing process poses a considerable challenge. The existing literature presents various parameters for predicting capping, yet a consensus has not been reached. This article addresses this gap by examining a diverse range of products (18 formulations, 8 of which exhibit capping) to forecast capping in biconvex tablets. The prediction is based on properties characterized from defect-free flat-faced tablets, all produced using identical process parameters (e.g., tensile strength, solid fraction, elastic recovery).
Single parameters and predictive indices from the literature were assessed across these formulations and were found inadequate for capping prediction. Subsequently, a predictive model was developed through decision tree analysis, relying on three in-die tablet properties: plastic energy per volume, in-die elastic recovery, and residual die-wall pressure. Testing this model on another set of 13 challenging formulations demonstrated its efficacy. The capping behavior of 29 out of the 31 formulations studied was accurately estimated using the developed model, with only two products predicted to cap that did not, showcasing the potential of this approach for effective risk analysis and assessment of capping occurrence.
Comments
No comments posted yet.
Add a comment