Scientific papers
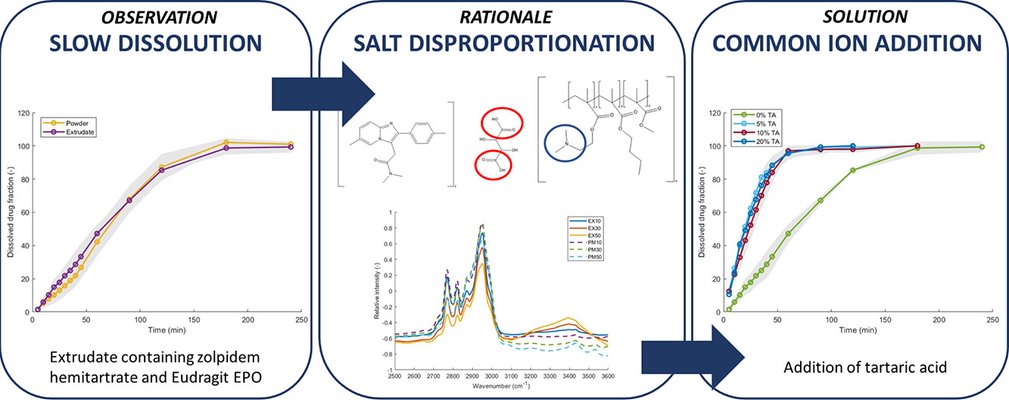
The present study aimed to optimize a previously developed non-clinical formulation for zolpidem deprescribing. The formulation under investigation comprises extruded mixtures of zolpidem hemitartrate (30% w/w) and Eudragit EPO (70% w/w), displaying suboptimal dissolution behavior. Tablets with identical target weight and solid fraction were produced by compressing both milled extrudates and physical mixtures. Initially, thermal and HPLC-DAD analyses were employed to identify the susceptibility of zolpidem hemitartrate to heat and shear degradation. The drug salt exhibited a tendency for thermally induced disproportionation, leading to an increase in impurity content post hot melt extrusion, albeit still adhering to ICH guidelines.
Subsequently, FTIR analysis of extrudates and physical mixtures revealed interactions and protonation of the dimethyl aminoethyl group from Eudragit EPO due to zolpidem disproportionation. This elucidation explained the formulations' slower dissolution kinetics compared to those containing non-ionizable polymers (e.g., Kollidon 12PF and Kollidon VA64). Finally, the addition of tartaric acid, serving as a microenvironmental pH modulator and common ion, emerged as a successful strategy to enhance dissolution kinetics. The incorporation of 5% tartaric acid led to a substantial increase in drug release after 15 minutes, from 10% to 40%. However, achieving immediate release behavior (80% within 15 minutes) was not yet accomplished.
Comments
No comments posted yet.
Add a comment














