Scientific papers
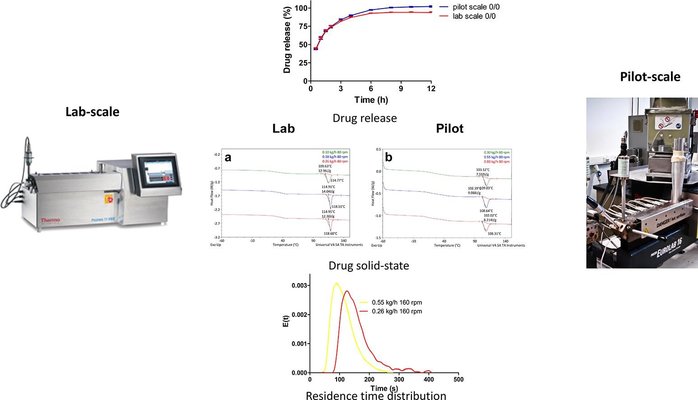
In this study, we evaluated the applicability of the volumetric scale-up law for transitioning from a laboratory-scale extruder (11 mm diameter) to a pilot-scale extruder (16 mm diameter) with geometric similarity, employing low feed rates (0.1–0.26 kg/h at the lab scale). A sustained release formulation was extruded at both scales using scaled feed rates based on the volumetric scale-up law. Various parameters, including specific mechanical energies, drug solid-state, drug dissolution, and responses related to residence time distribution (such as axial mixing degree, mean residence time, and width of distribution), were compared between the two scales. The results indicated that the difference in mean residence time between the two scale extruders decreased with higher throughput and fill level. Overall, specific mechanical energies (SME) were found to be comparable between scales when applying the volumetric scale-up law (i.e., using a scaling factor q = 3) and precisely matching with a scaling factor of q = 2.6. Additionally, it was observed that plug flow conditions at the lab scale should be avoided before scaling up to achieve similar SMEs. At a scaling factor of q = 2, the same degree of axial mixing (represented by the Peclet number) was demonstrated. If drug solid-state is a critical quality attribute (CQA), the focus should be on the screw speed and cooling capacity of the larger scale extruder. Drug dissolution exhibited similarity between scales and remained independent of drug solid-state for this formulation, suggesting successful scale-up feasibility.
Comments
No comments posted yet.
Add a comment













