Scientific papers
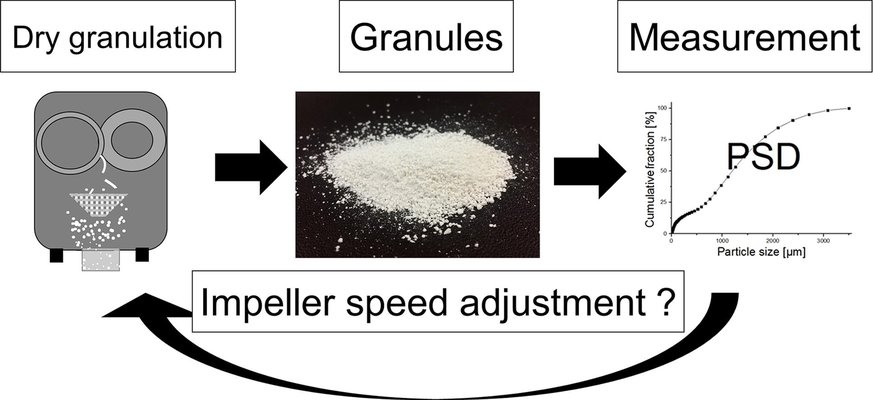
As the continuous manufacturing of solid pharmaceuticals advances, there is a growing interest in adopting continuous granulation methods. To ensure the monitoring of product quality and enforce control strategies, the integration of process analytical technology tools becomes crucial.
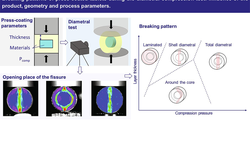
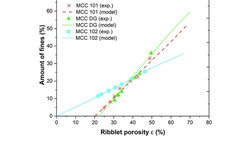
Three individual materials commonly employed in dry granulation, along with two formulations—one containing ibuprofen and the other acetaminophen—were processed at varying process parameters, each exhibiting distinct compaction and fracture behaviors. A statistical analysis was conducted to determine the influence of process parameters and identify those suitable for a granule size control approach in continuous dry granulation. The specific compaction force and impeller speed were identified as significant factors in each design of the experiment. However, the impeller speed was deemed the sole appropriate parameter for controlling granule size, as its impact on granule density is unlikely. Nevertheless, certain constraints, such as an upper impeller speed limit to prevent excessive fines and a lower limit to avoid a reduction in throughput, must be considered. Additionally, a decrease in median granule size was noted at higher throughputs for plastically deforming materials and formulations.
Comments
No comments posted yet.
Add a comment