Scientific papers
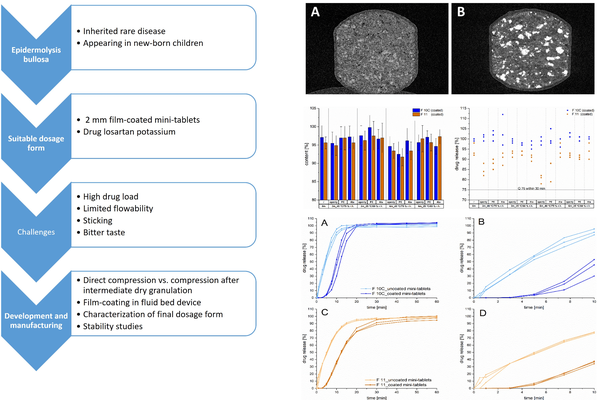
Epidermolysis bullosa is a genetically heterogeneous skin fragility disorder that manifests with multiorgan involvement, often evident in newborn children. The condition leads to severe progressive fibrosis following skin blistering, mucosal lesions, and impaired wound healing, ultimately increasing the risk of developing highly aggressive squamous cell carcinomas. Losartan potassium (LP) has demonstrated positive effects, prompting clinical interest in developing 2 mm mini-tablets with LP for treating affected children.
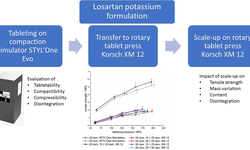
The development process faced several challenges: initial tableting experiments revealed limited flowability and sticking to punches due to a high drug load, and the LP was reported to have a bitter taste. Sticking to punches was mitigated by incorporating SMCC 50 and a combination of different lubricants. However, direct compression trials on a Korsch XM 12 rotary press faced complications due to compaction phenomena in the hopper. Consequently, an intermediate dry granulation method was successfully introduced. Two final formulations of the mini-tablets met European Pharmacopoeia requirements for disintegration times (<15 min) and friability (<1.0%). The mean tensile strengths were around 1 MPa, striking a balance between manufacturability and sufficient mechanical strength for subsequent coating studies.
The subsequent coating step effectively delayed the initial drug release for over 2 minutes. The coated mini-tablets achieved an acceptance value ≤15, and stability studies indicated a promising shelf life.
Comments
No comments posted yet.
Add a comment