Scientific papers
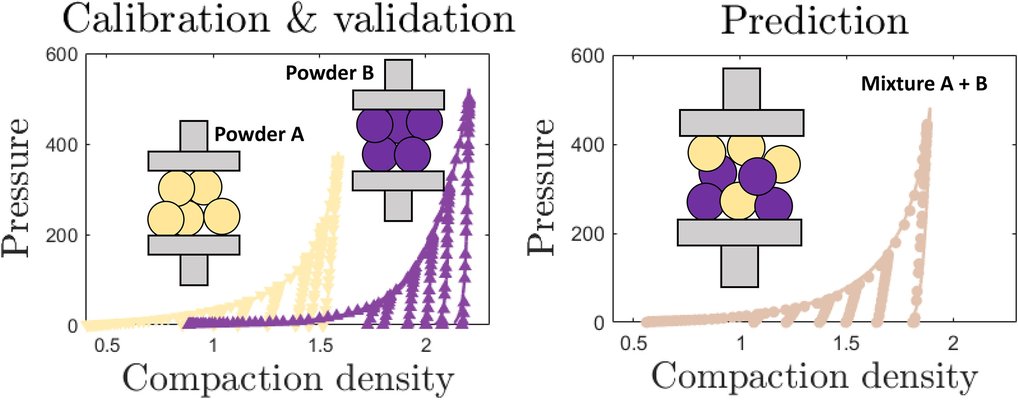
In the pharmaceutical industry, the formulation of tablets remains a challenging task despite the widespread adoption of solid-form drug delivery. This challenge arises from the strong dependence of tablet properties on both powder composition and the intricacies of the compaction process. Powder compaction simulations, employing the finite element method (FEM) alongside the density-dependent Drucker-Prager Cap model, offer a valuable tool for facilitating the design of pharmaceutical tablets. However, the typical manual parametrization process, which necessitates experimental data for each considered powder, becomes cumbersome when dealing with various ratios of component powders.
To address this, an automated parameterization workflow was developed and validated using experimental powder mixtures of micro-crystalline cellulose and dibasic calcium phosphate dihydrate. The FEM simulations successfully replicated experimental compaction curves, exhibiting a mean error of 2.5% of the maximum compaction pressure. Additionally, a mixing methodology was devised to estimate parameters for mixtures using only pure-component parameters as input. This approach predicted the experimental compaction curves of mixtures with a mean error of 4.8%.
Comments
No comments posted yet.
Add a comment